Benchmarking Foundation Models and Parameter-Efficient Fine-Tuning for Prognosis Prediction in Medical Imaging (2506.18434v1)
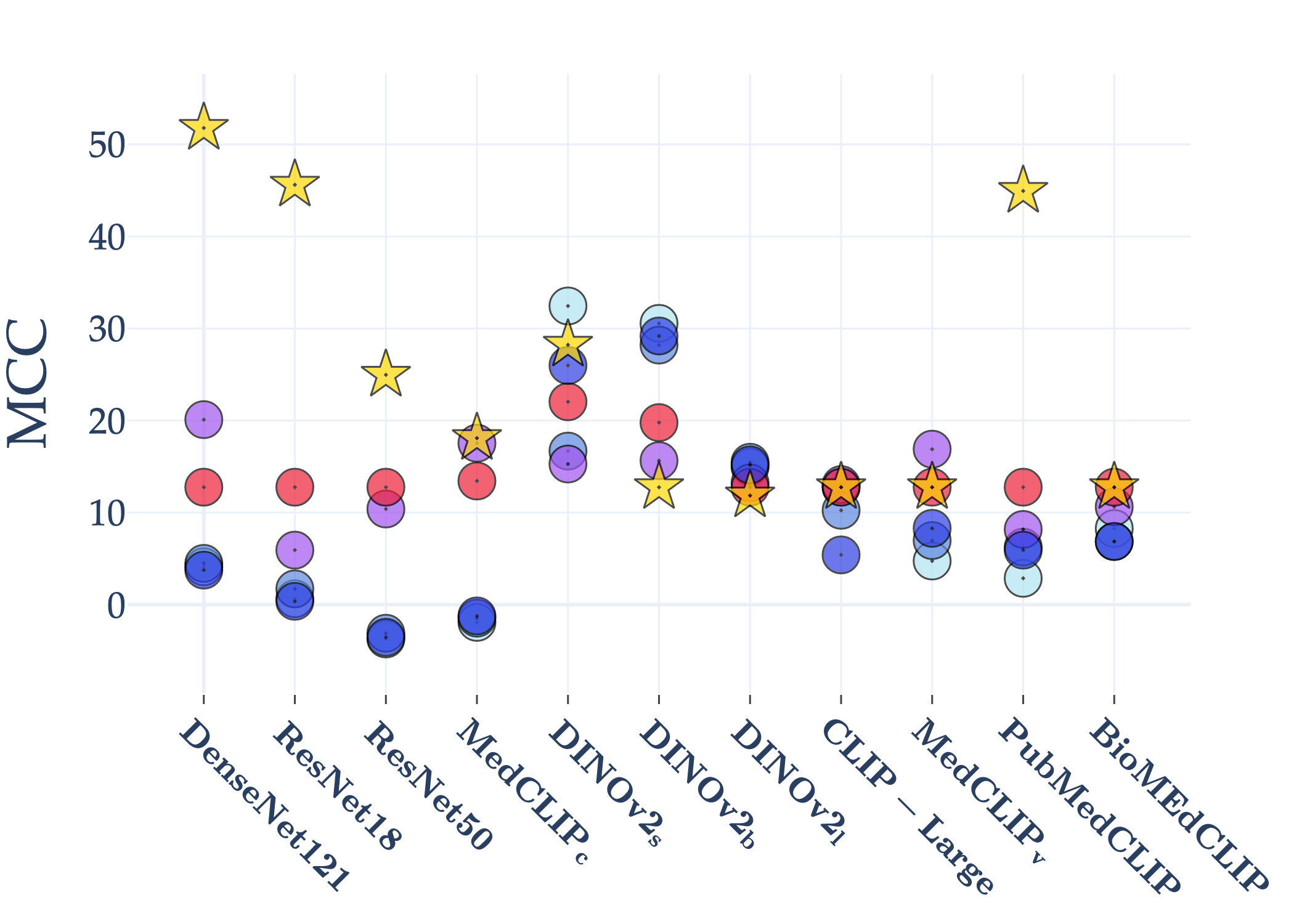
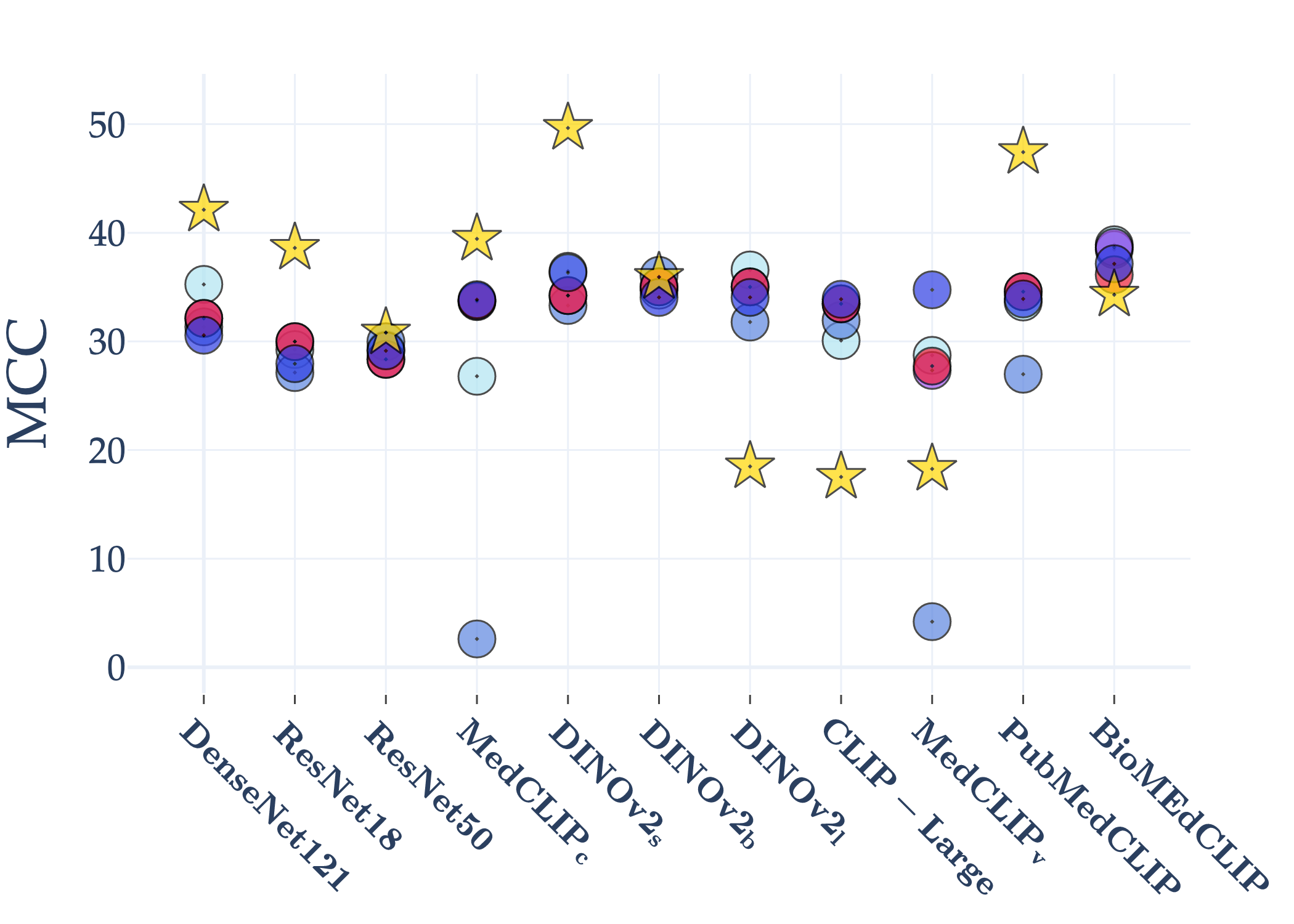
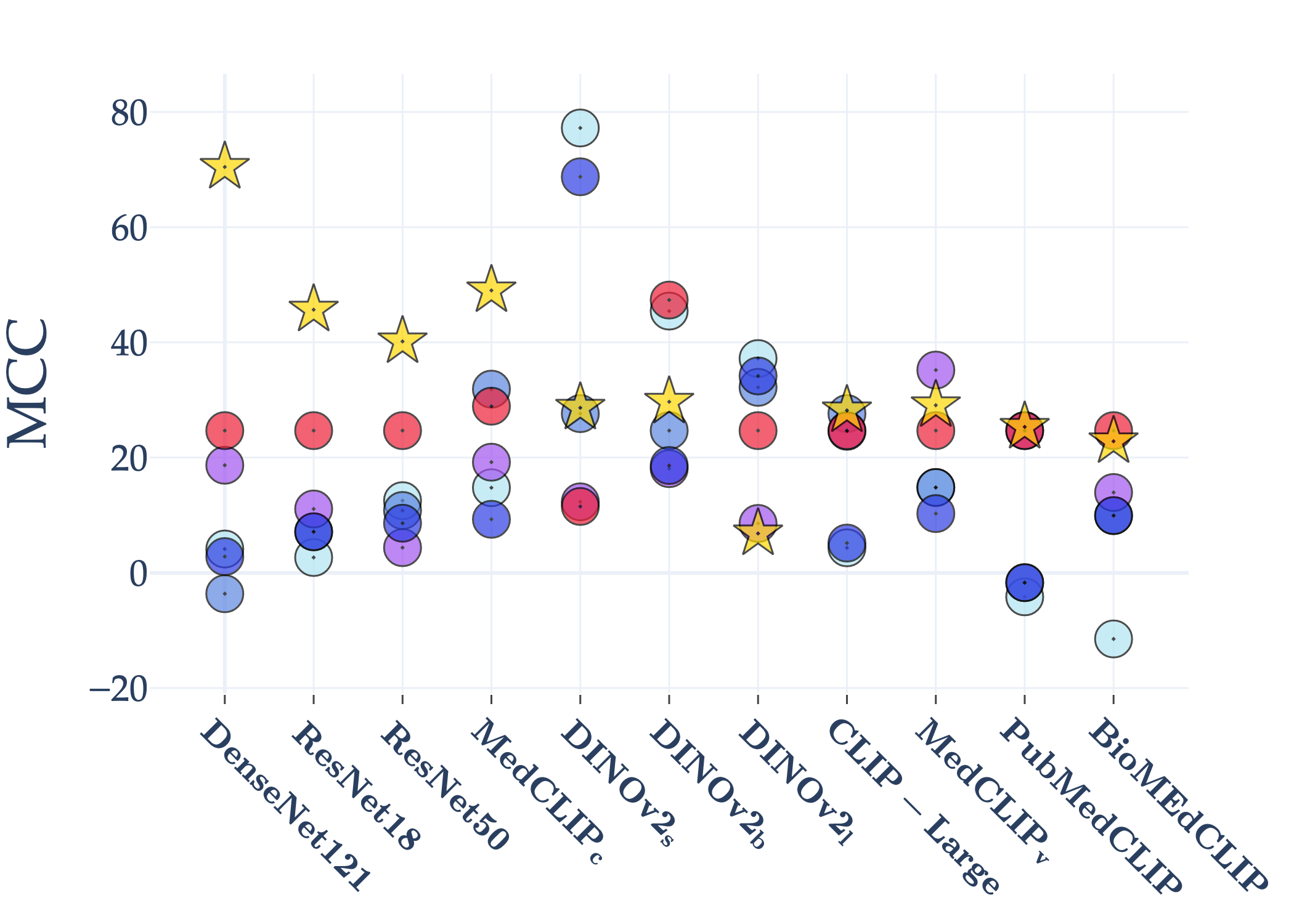
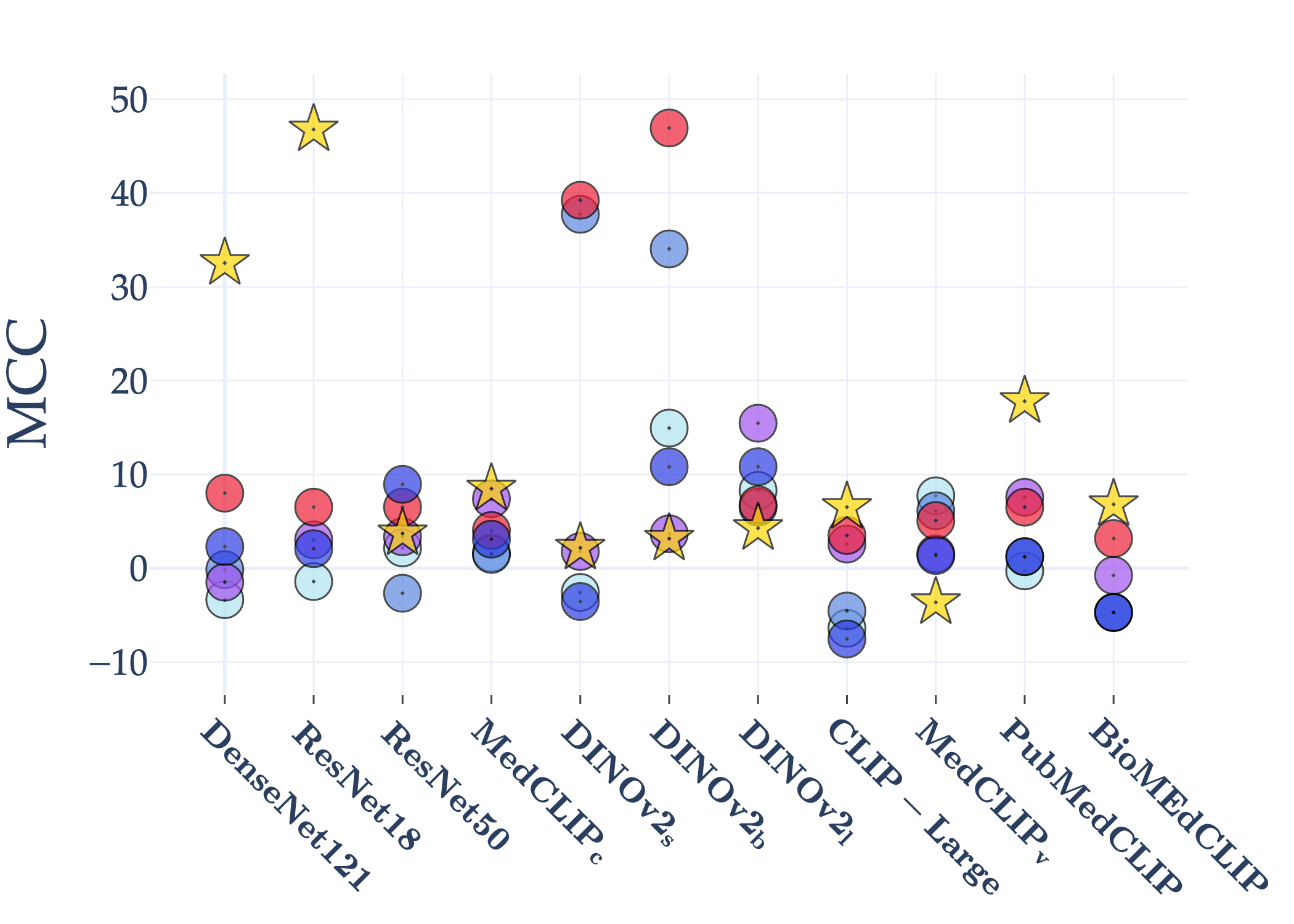
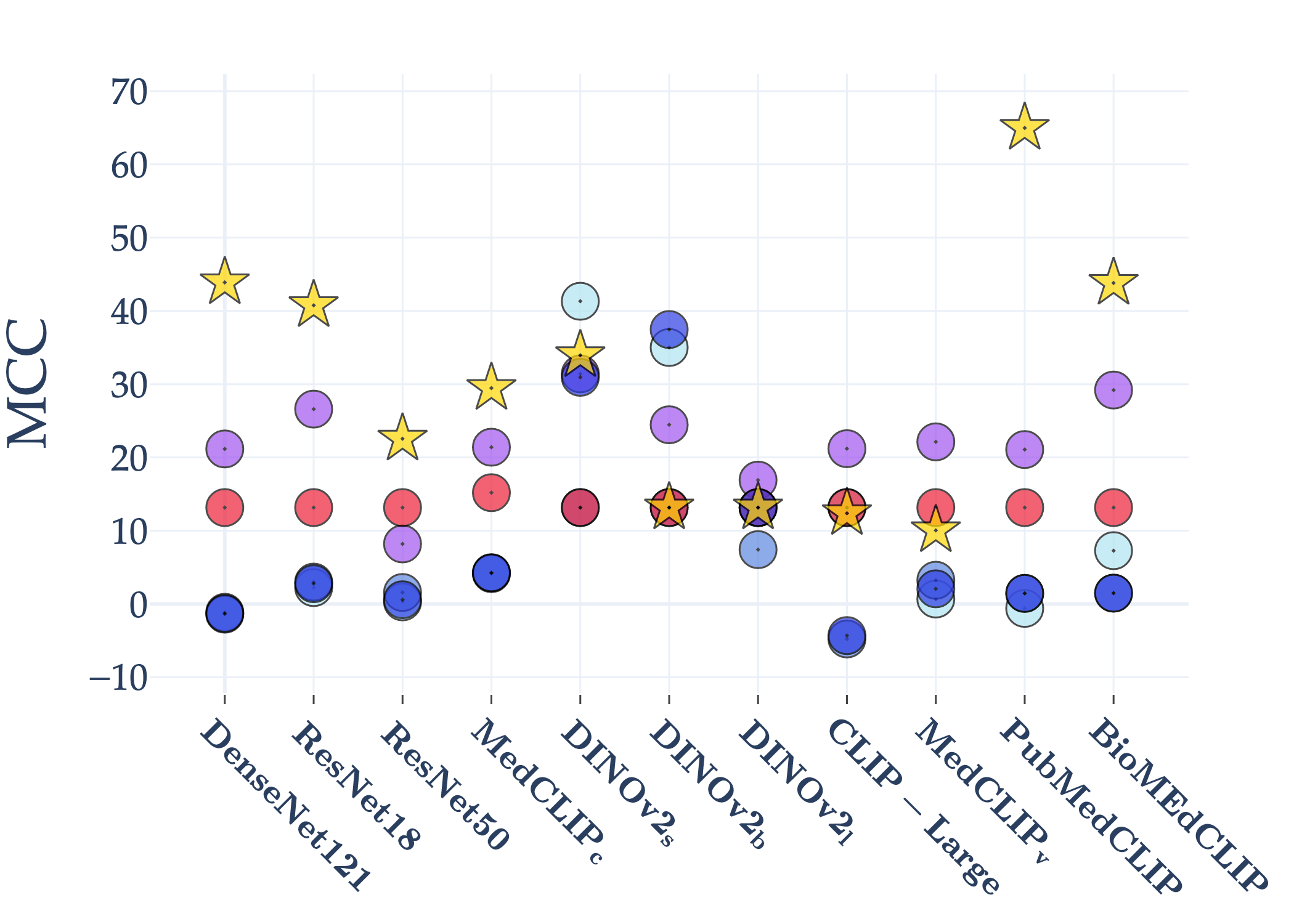
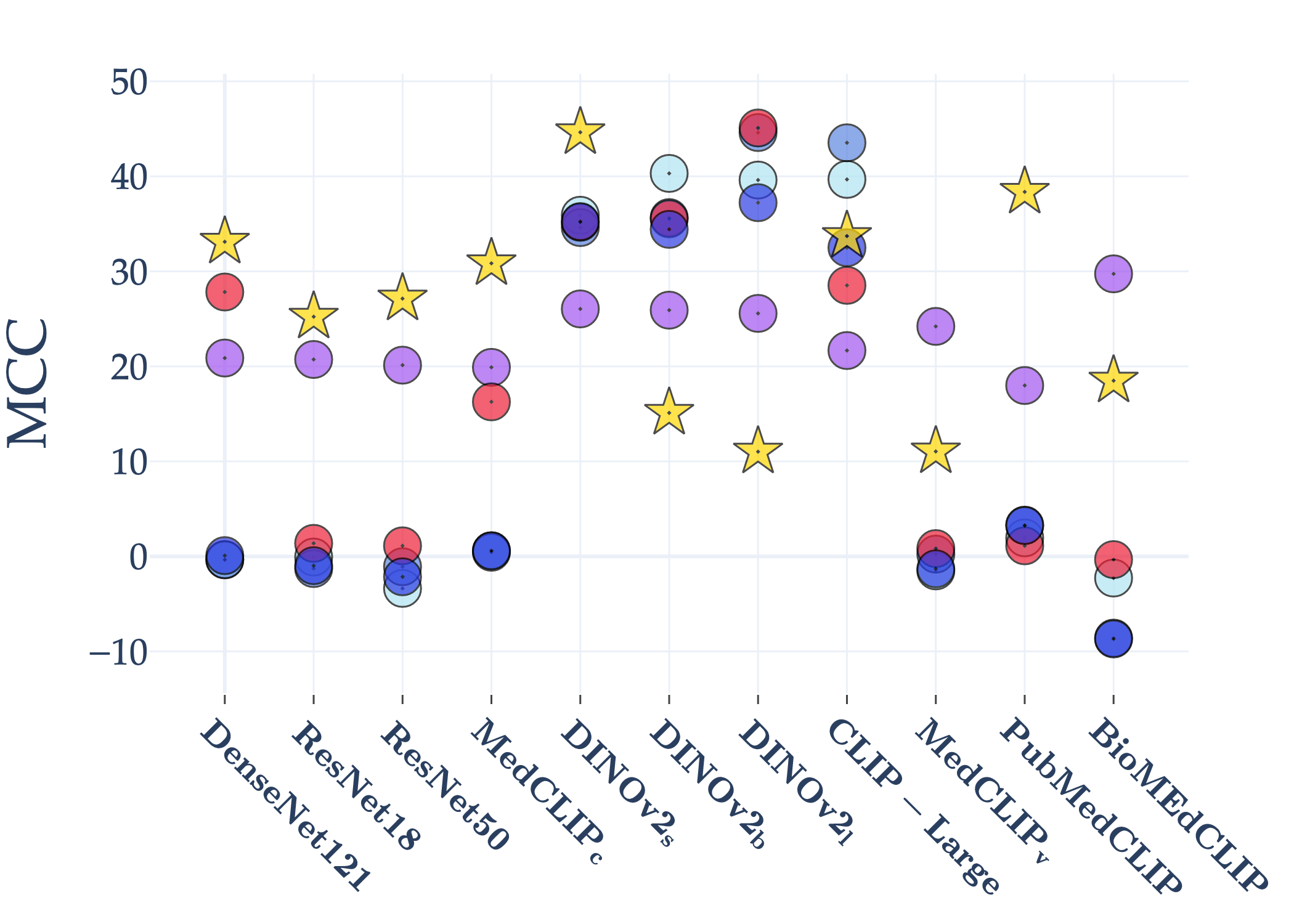
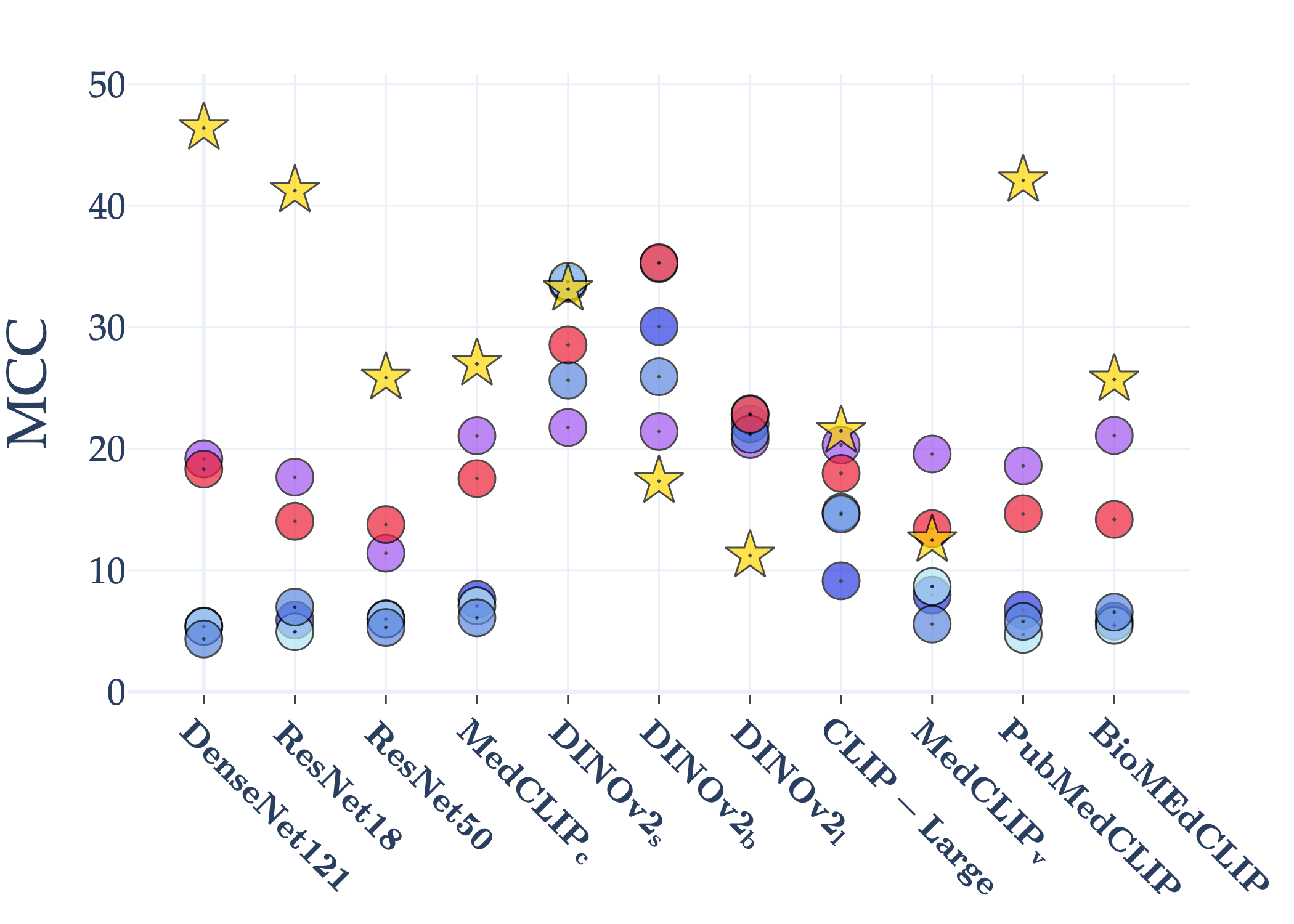
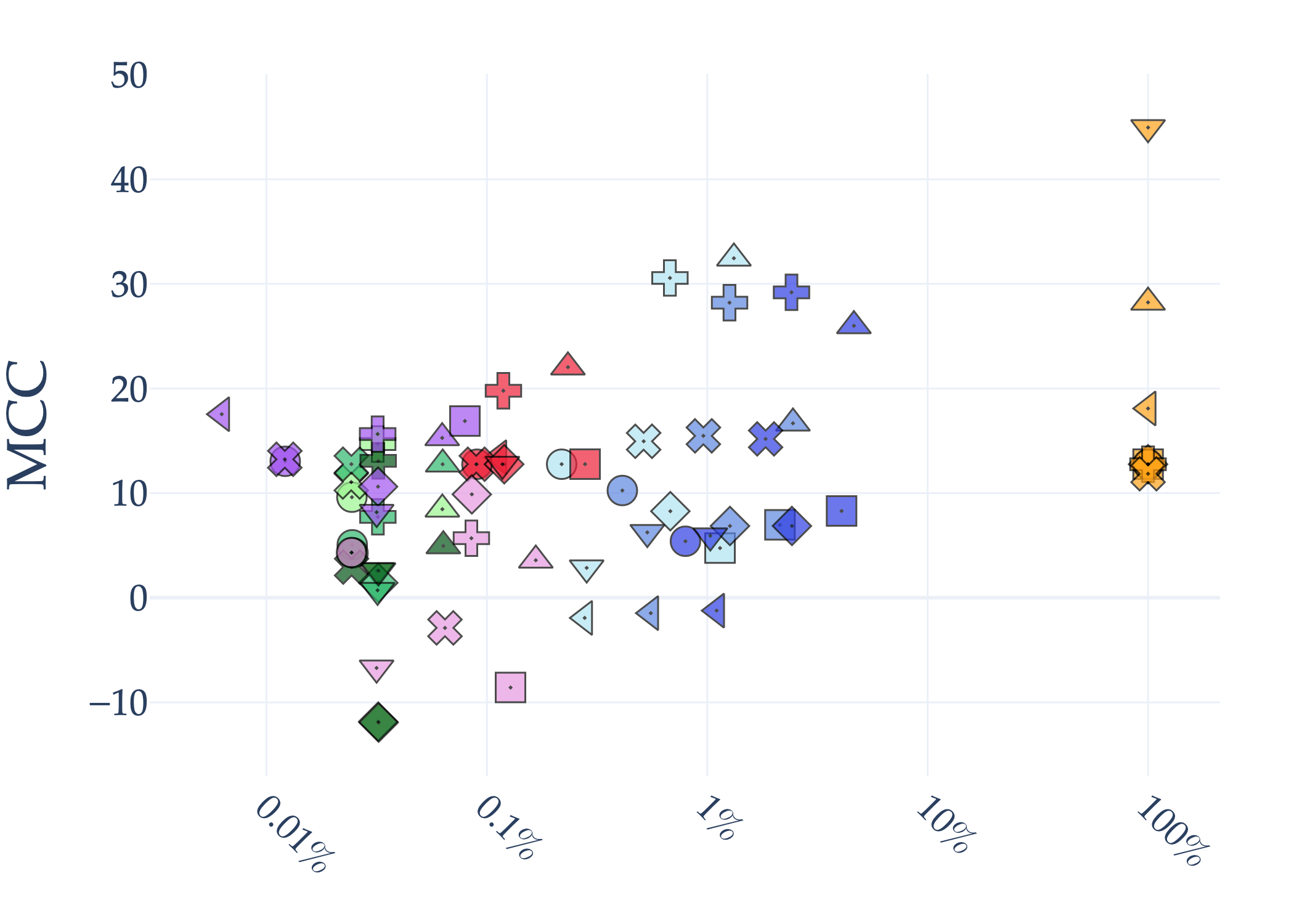
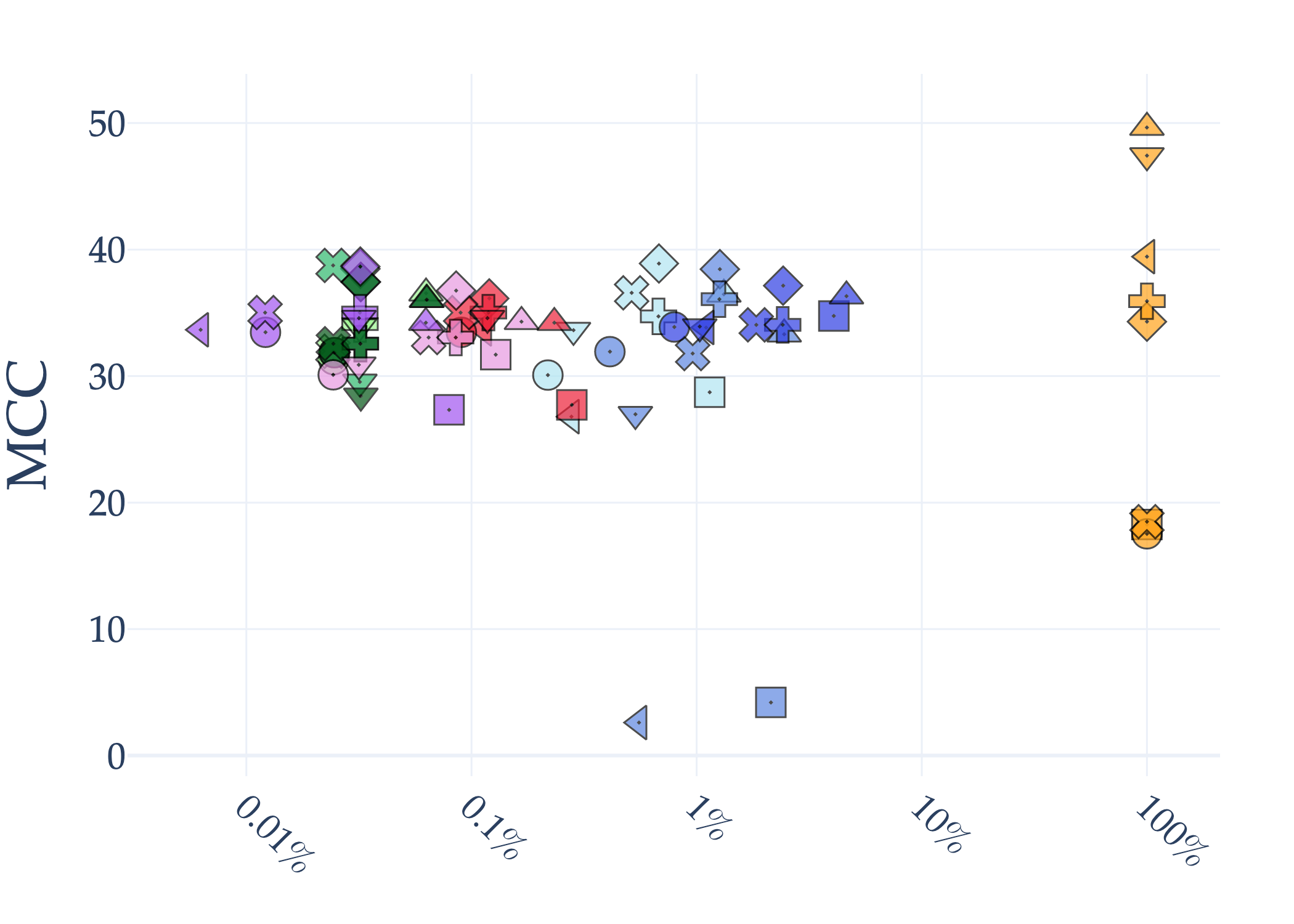
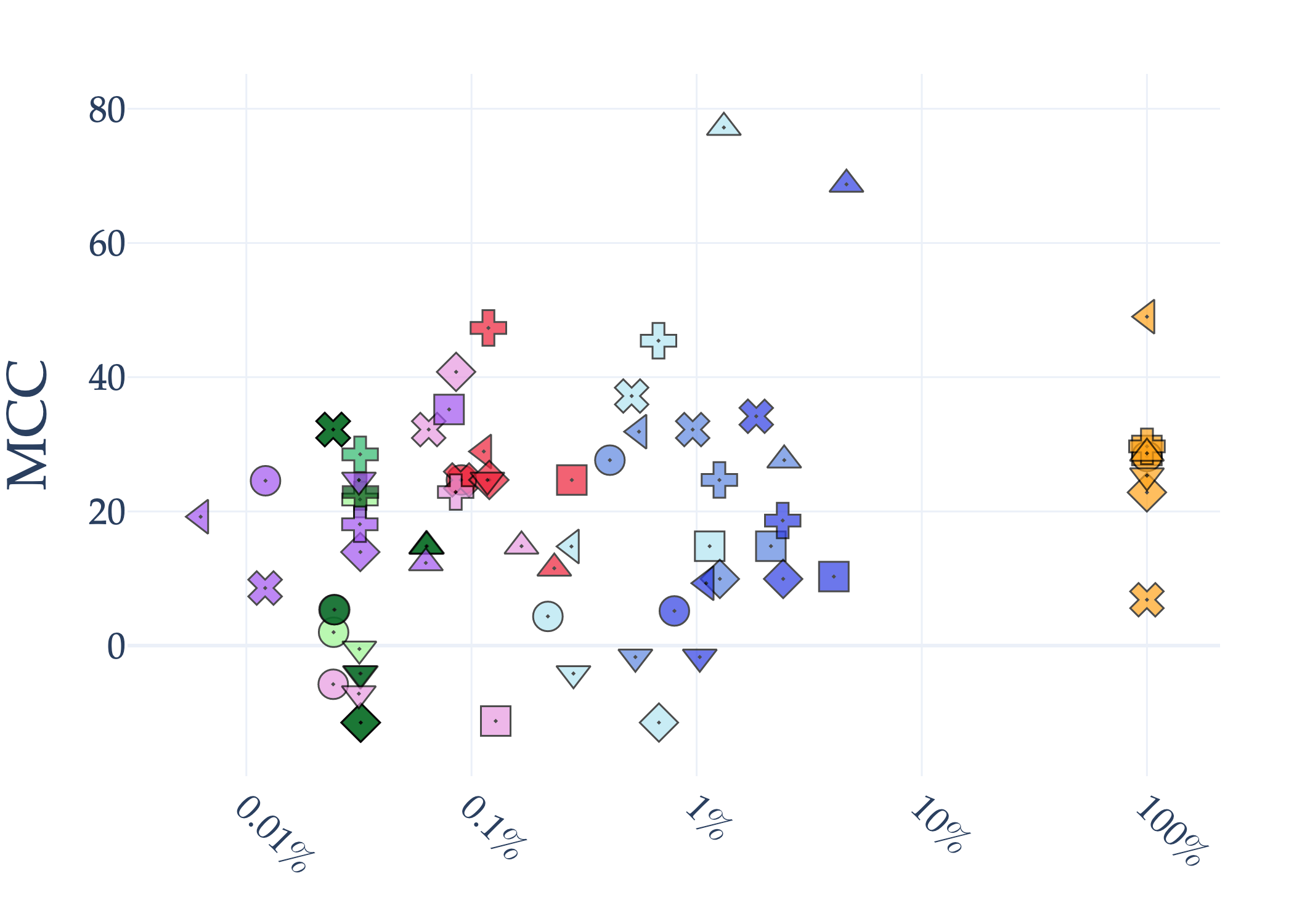
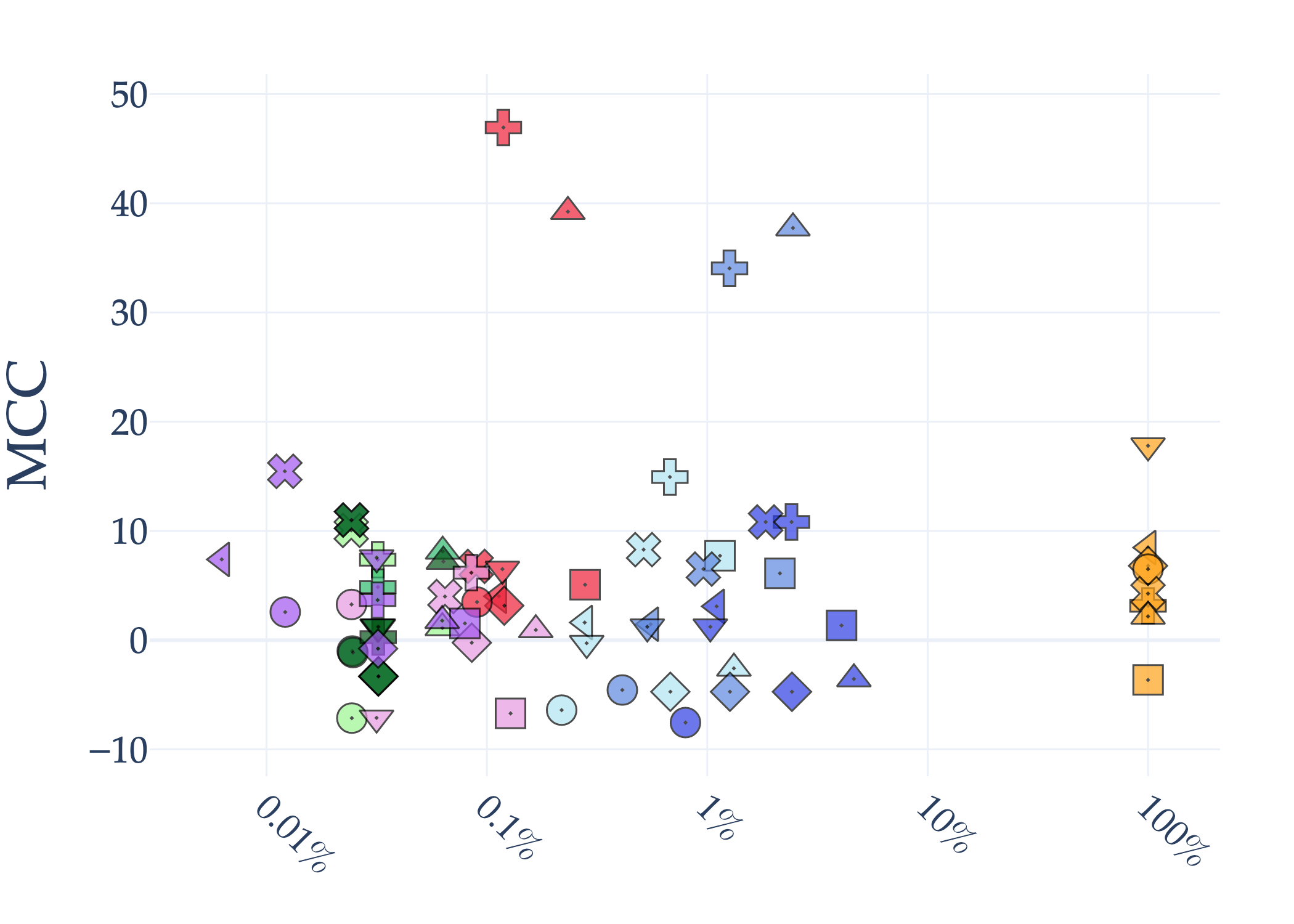
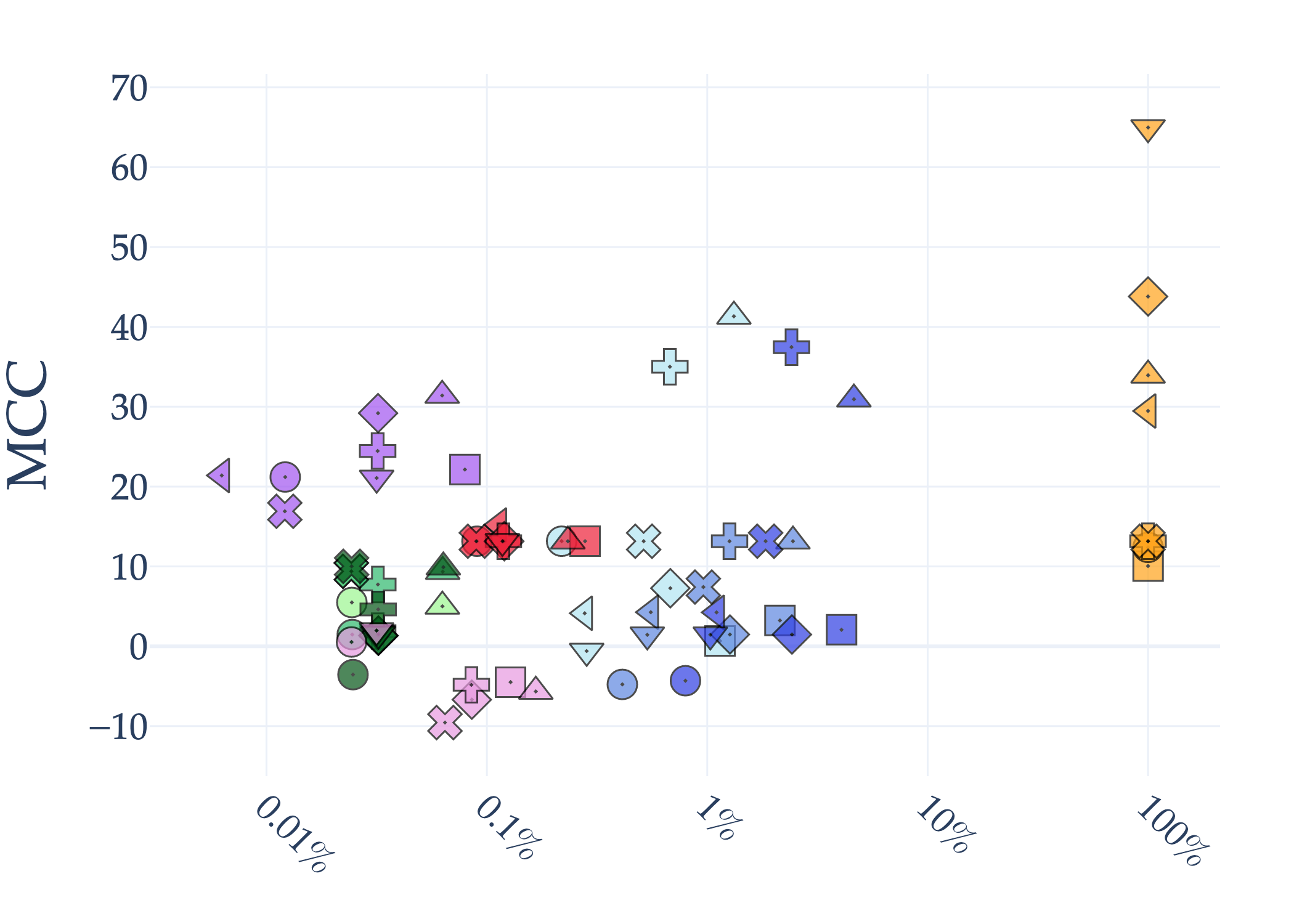
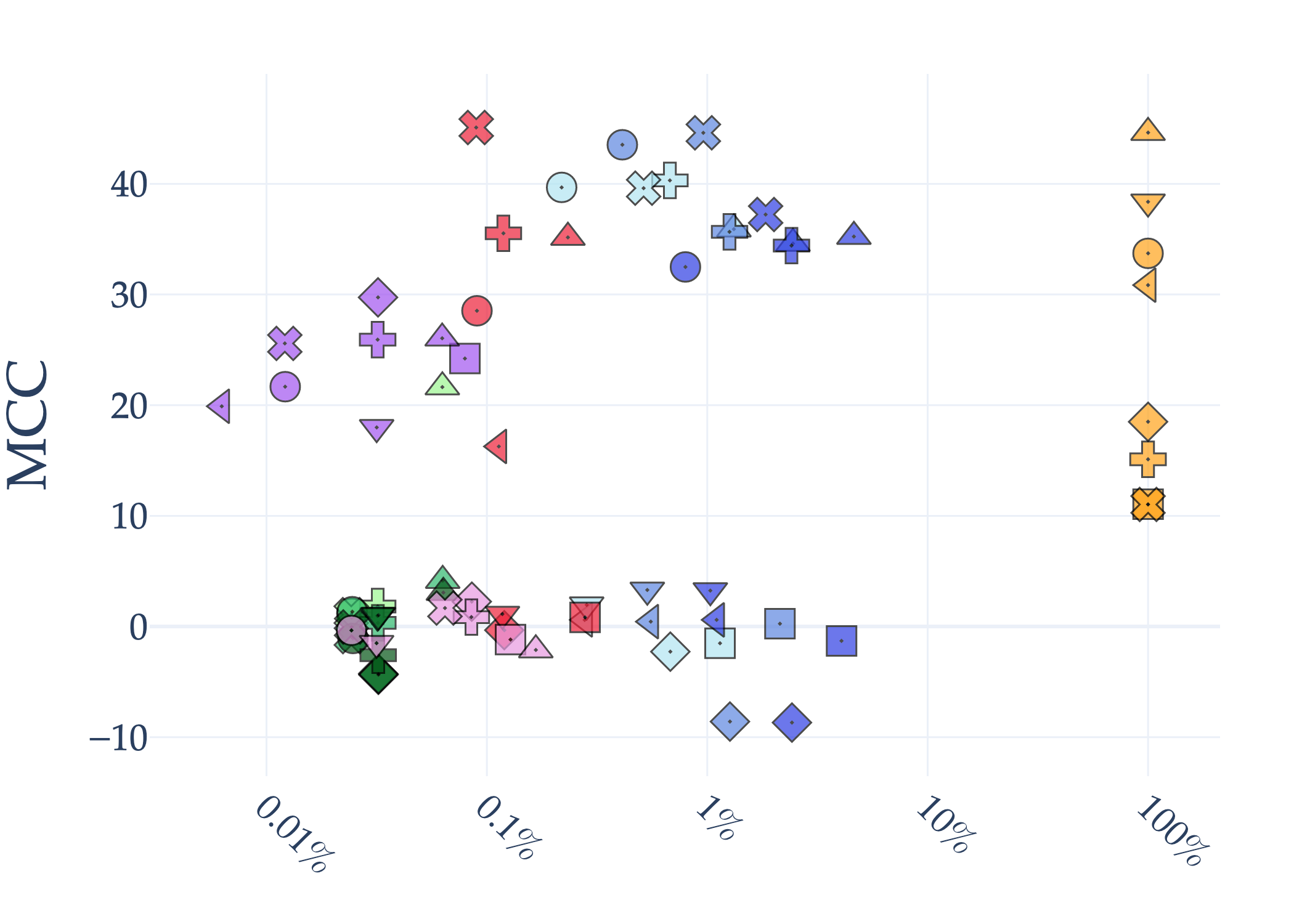
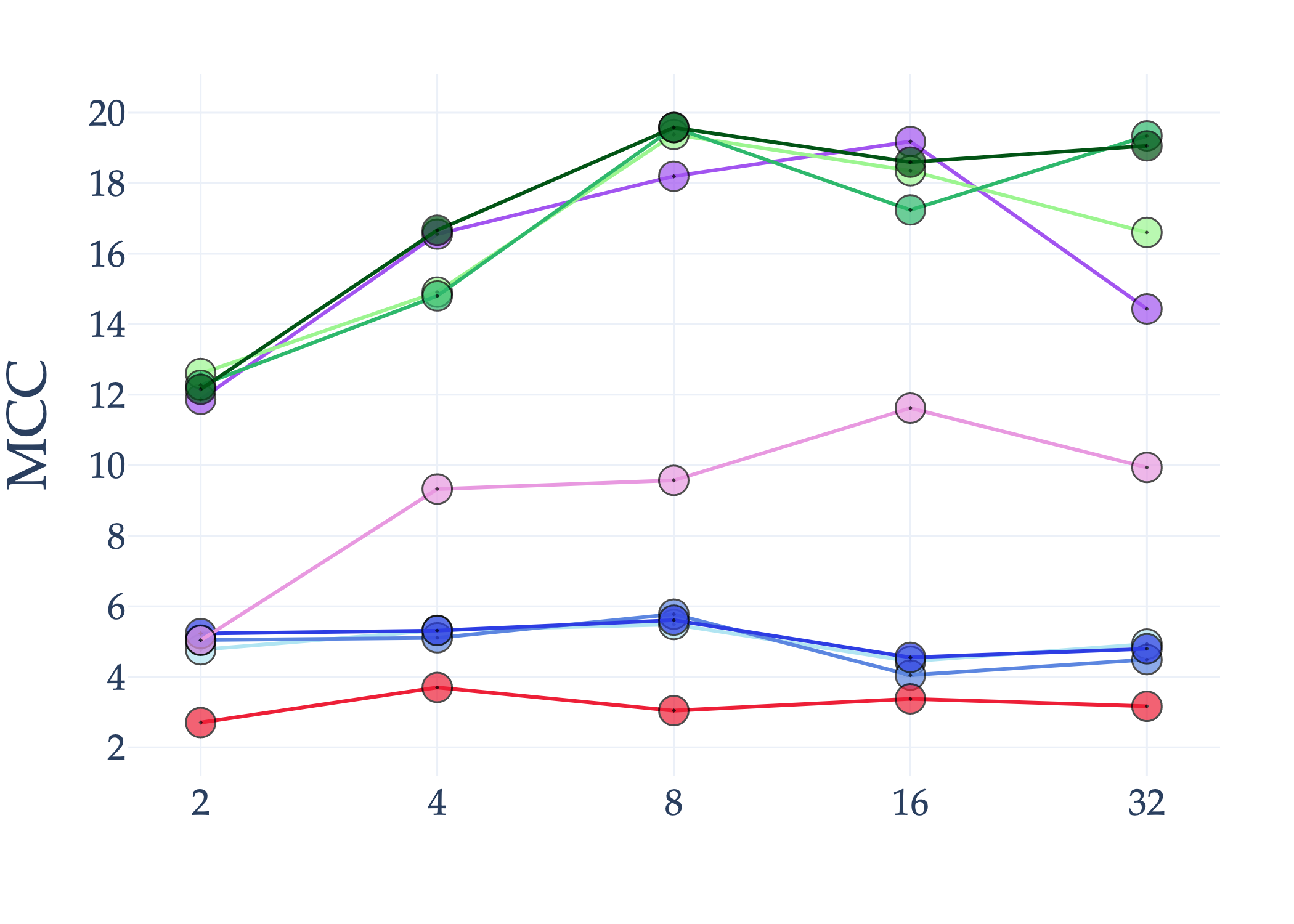
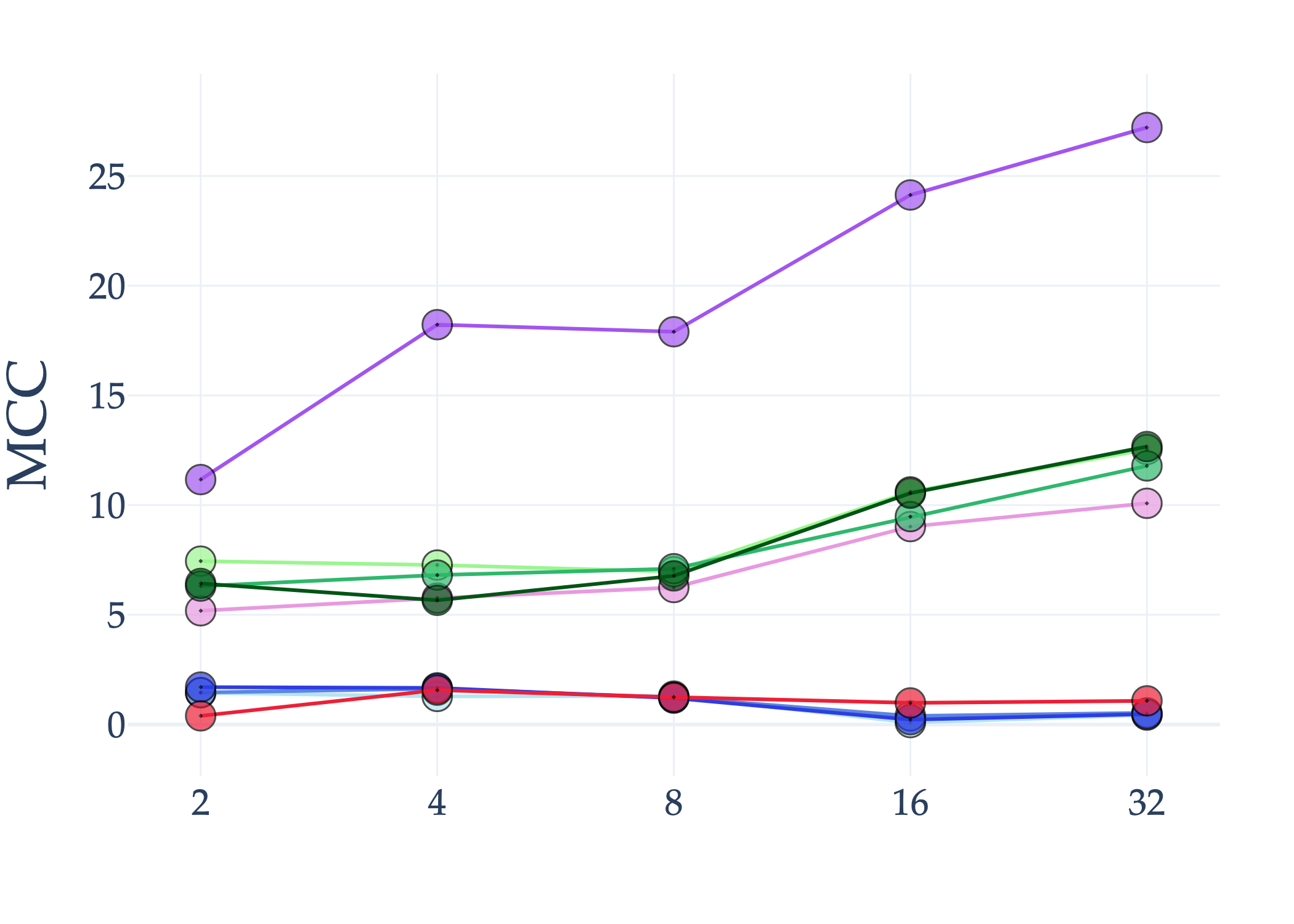
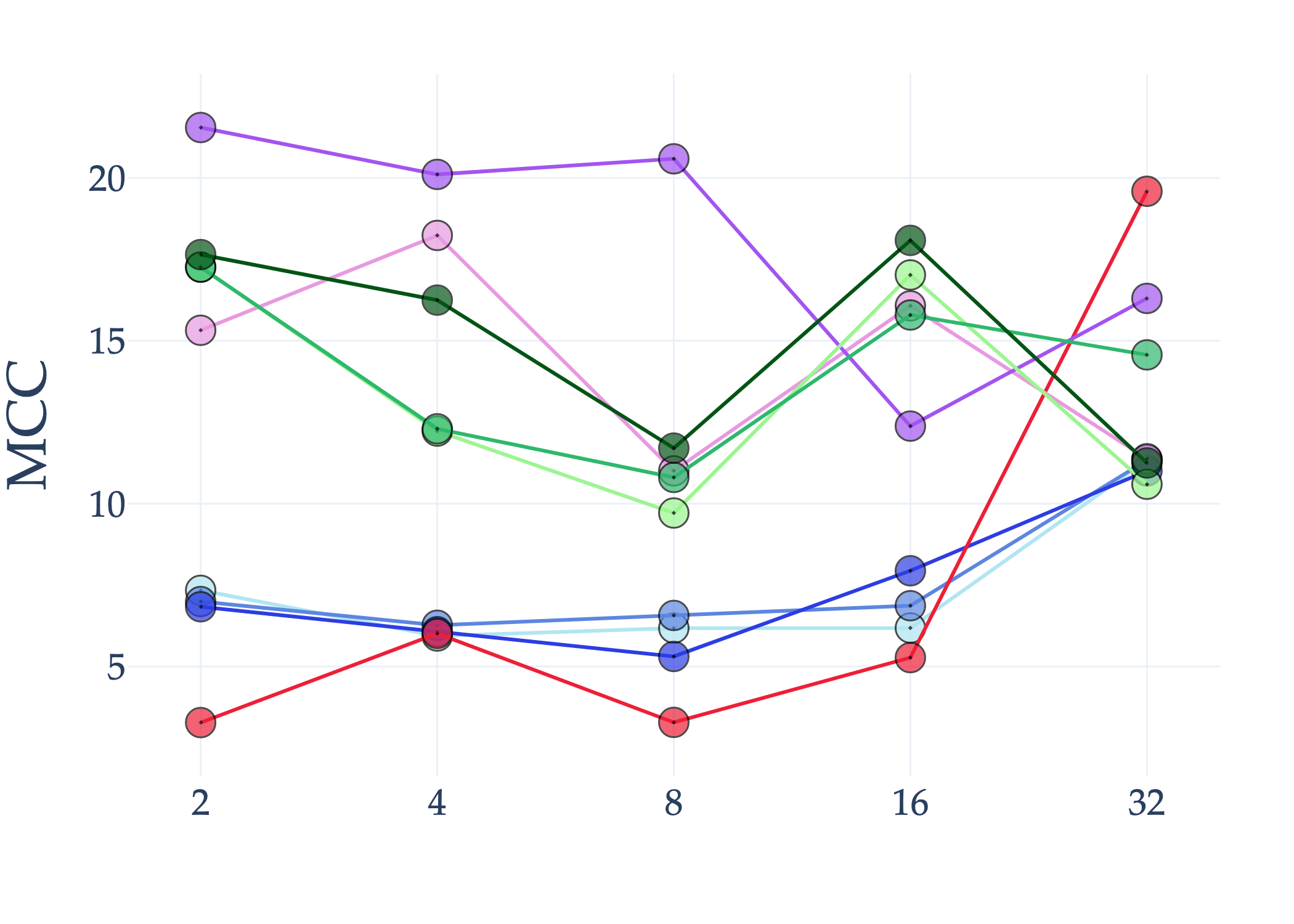
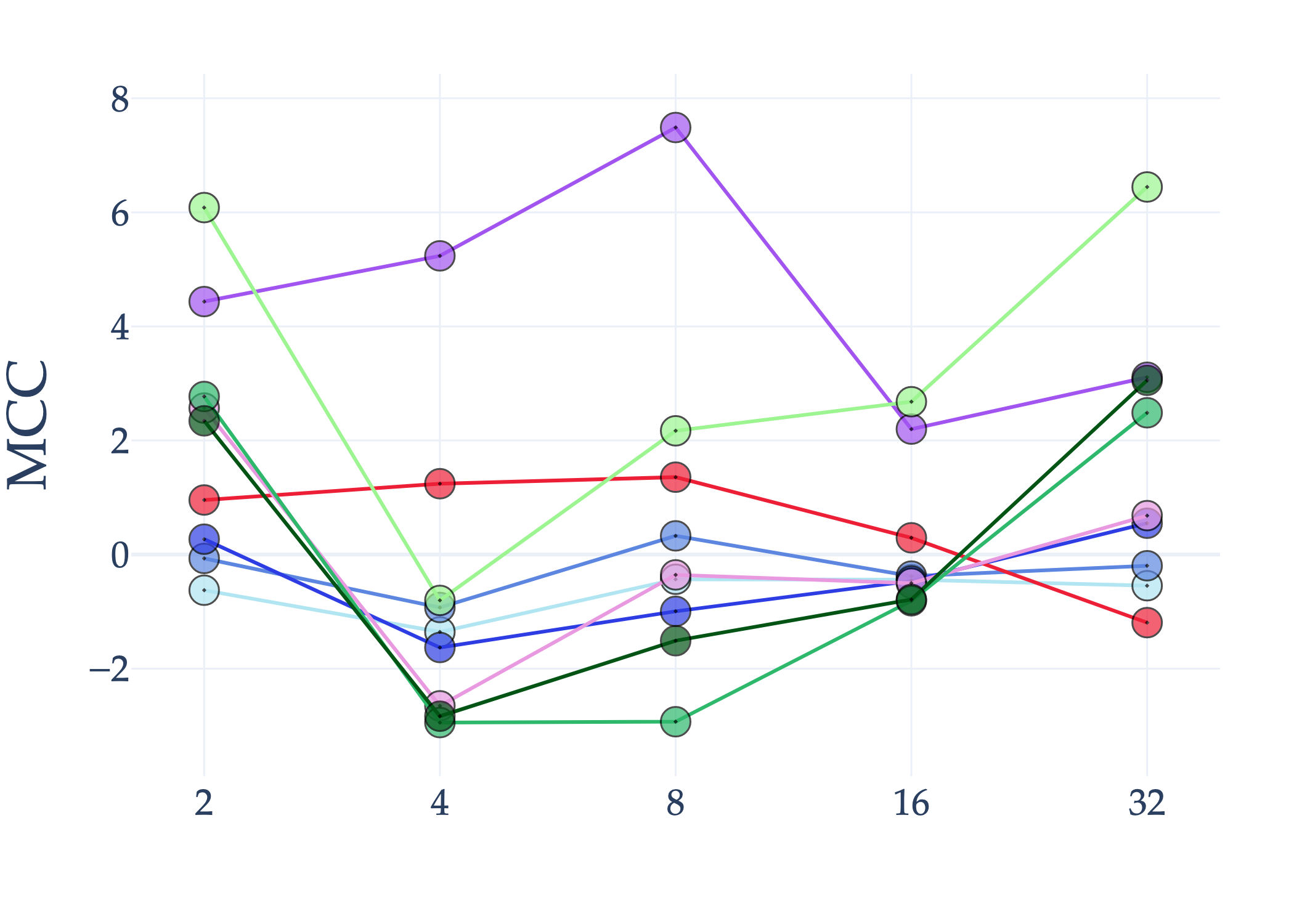
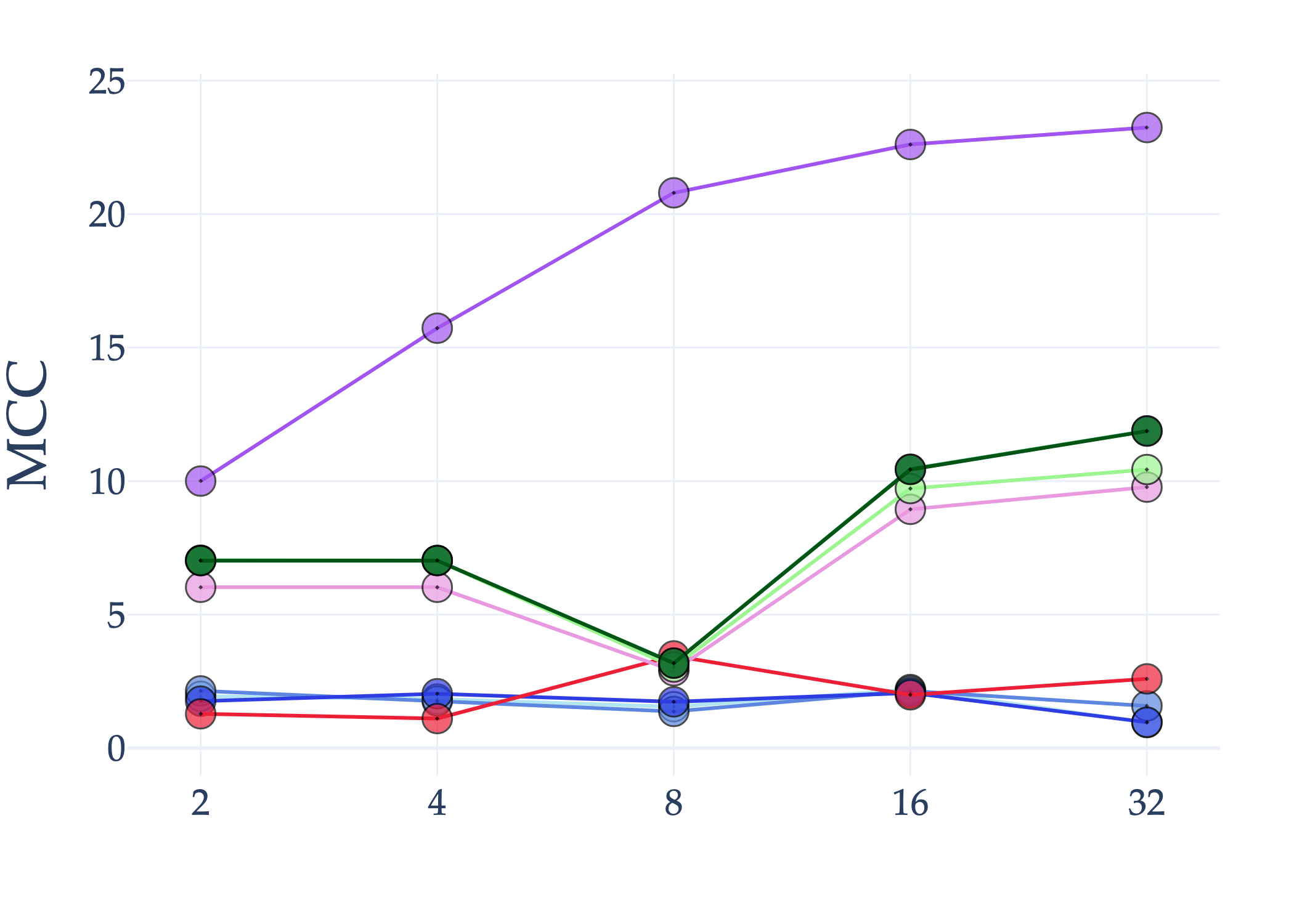
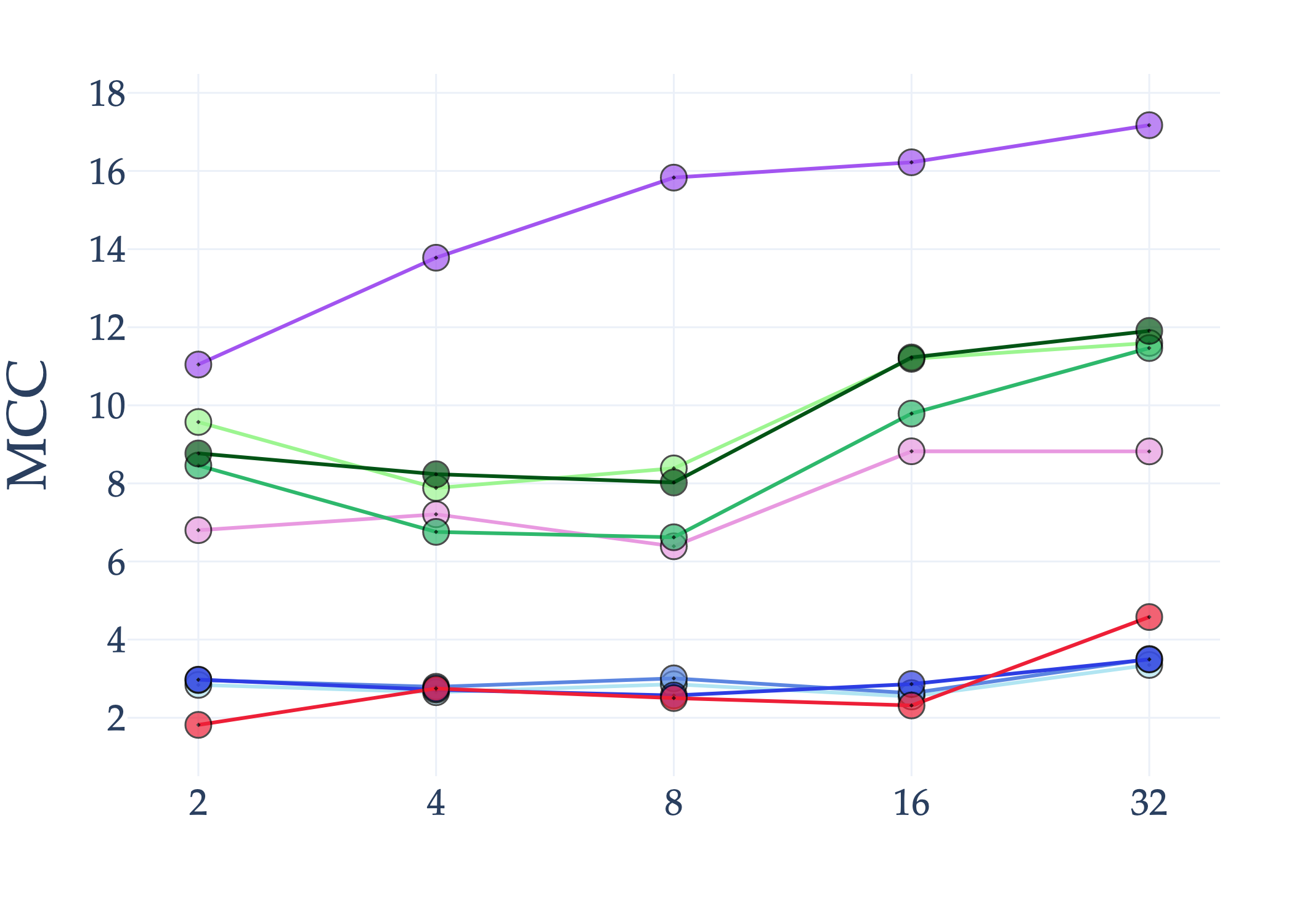
Abstract: AI holds significant promise for improving prognosis prediction in medical imaging, yet its effective application remains challenging. In this work, we introduce a structured benchmark explicitly designed to evaluate and compare the transferability of Convolutional Neural Networks and Foundation Models in predicting clinical outcomes in COVID-19 patients, leveraging diverse publicly available Chest X-ray datasets. Our experimental methodology extensively explores a wide set of fine-tuning strategies, encompassing traditional approaches such as Full Fine-Tuning and Linear Probing, as well as advanced Parameter-Efficient Fine-Tuning methods including Low-Rank Adaptation, BitFit, VeRA, and IA3. The evaluations were conducted across multiple learning paradigms, including both extensive full-data scenarios and more clinically realistic Few-Shot Learning settings, which are critical for modeling rare disease outcomes and rapidly emerging health threats. By implementing a large-scale comparative analysis involving a diverse selection of pretrained models, including general-purpose architectures pretrained on large-scale datasets such as CLIP and DINOv2, to biomedical-specific models like MedCLIP, BioMedCLIP, and PubMedCLIP, we rigorously assess each model's capacity to effectively adapt and generalize to prognosis tasks, particularly under conditions of severe data scarcity and pronounced class imbalance. The benchmark was designed to capture critical conditions common in prognosis tasks, including variations in dataset size and class distribution, providing detailed insights into the strengths and limitations of each fine-tuning strategy. This extensive and structured evaluation aims to inform the practical deployment and adoption of robust, efficient, and generalizable AI-driven solutions in real-world clinical prognosis prediction workflows.
Sponsor
Paper Prompts
Sign up for free to create and run prompts on this paper using GPT-5.
Top Community Prompts
Collections
Sign up for free to add this paper to one or more collections.