- The paper introduces BrainSynth, a two-stage diffusion model that uses metadata like age and sex to synthesize anatomically plausible 3D brain MRIs.
- The model demonstrates strong anatomical consistency with real MRIs, with evaluations using Cohen’s d and Pearson’s r showing significant alignment in sex differences and aging effects.
- Integrating synthetic MRIs into CNN training improves age-prediction accuracy, highlighting the practical benefits for augmenting neuroimaging datasets.
Introduction
The paper "Metadata-Conditioned Generative Models to Synthesize Anatomically-Plausible 3D Brain MRIs" (2310.04630) introduces a novel approach to generating high-resolution synthetic brain MRIs using generative AI models. These models address the challenges faced by previous attempts which primarily focused on optimizing visual quality without consideration for the anatomical relevance critical to neuroscience. By implementing a diffusion model named BrainSynth, the research achieves state-of-the-art visual quality and evaluates the anatomical plausibility of synthesized MRIs with respect to macrostructural properties and effects of age and sex.
BrainSynth Model Architecture
The BrainSynth model is a two-stage Diffusion Probabilistic Model (DPM) designed to synthesize MRIs conditioned on metadata such as age and sex. Stage I employs Vector Quantization coupled with a Variational Autoencoder (VQ-VAE) to map MRIs onto a quantized vector space derived from a codebook. This stage facilitates the representation of MRIs in a more stable and accessible format, avoiding the collapse issues prevalent in GANs.

Figure 1: Architecture of BrainSynth: Stage I consists of a VQ-VAE reducing the real 3D MRI to a quantized vector representation derived from a code book.
Stage II further isolates metadata-free residuals and applies a transformer-based discrete diffusion model to impose conditional masking, which learns the distribution of these vector representations within the training data. This facilitates the synthesis of new MRIs by recomposing random residual codes with metadata-specific encodings.
Evaluation and Results
The pivotal component of the paper is a novel procedure that quantifies anatomical plausibility through evaluation frameworks. This process involves comparing synthesized MRIs against real MRIs in terms of their regional anatomical properties and yields metrics such as Cohen's d for sex differences and Pearson's r for aging effects. Results show that more than half of the synthetic MRIs achieved small effect sizes, indicative of high anatomical accuracy in numerous brain regions, though some regions with higher geometric complexity reflected greater discrepancies.
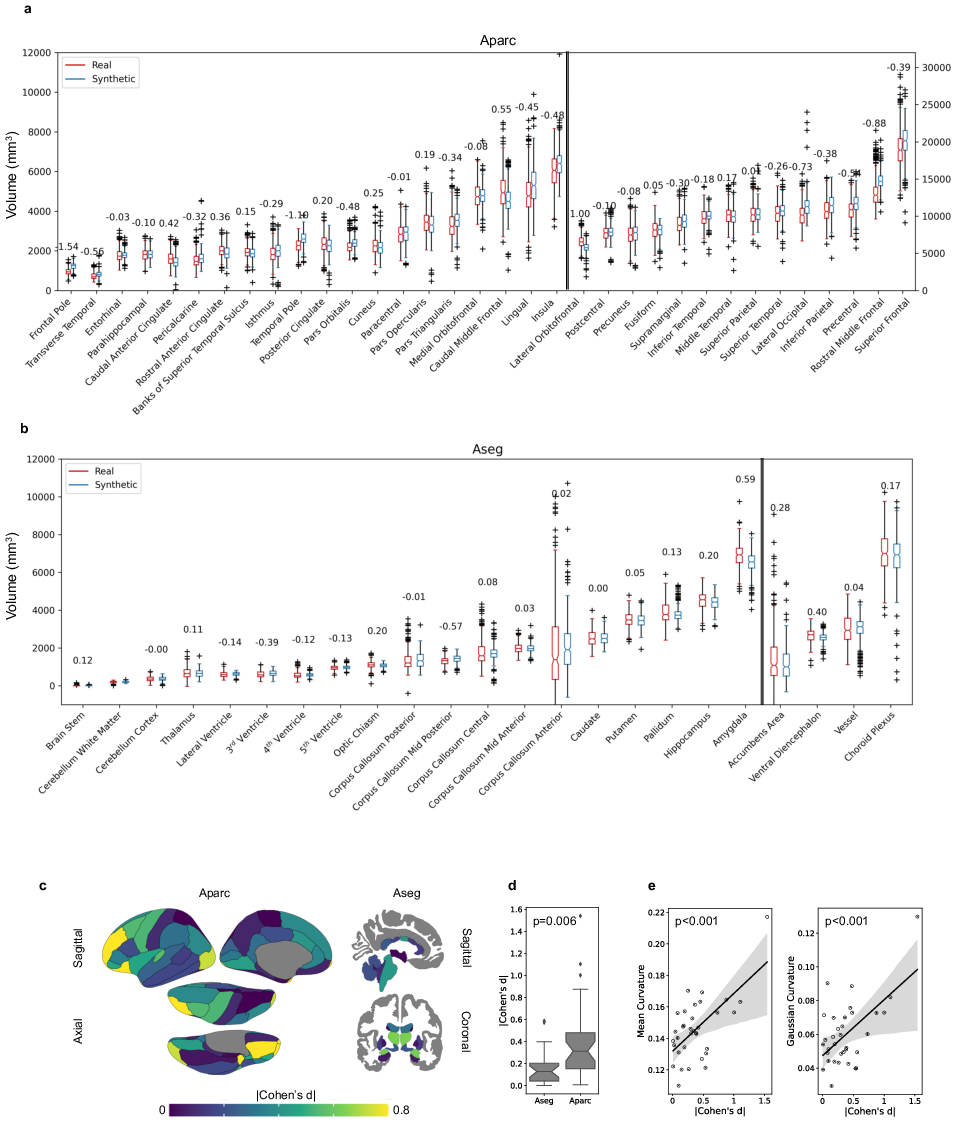
Figure 2: Distribution of the volume for each of the (a) 34 cortical regions (i.e., Aparc) and (b) 23 subcortical regions in real and synthesized MRIs with effect sizes denoting anatomical plausibility.
Sex differences and aging effects inherent in real MRIs were shown to be reproducible in synthetic MRIs, with Pearson correlation coefficients illustrating significant alignment between synthetic and real data across the anatomical structure.
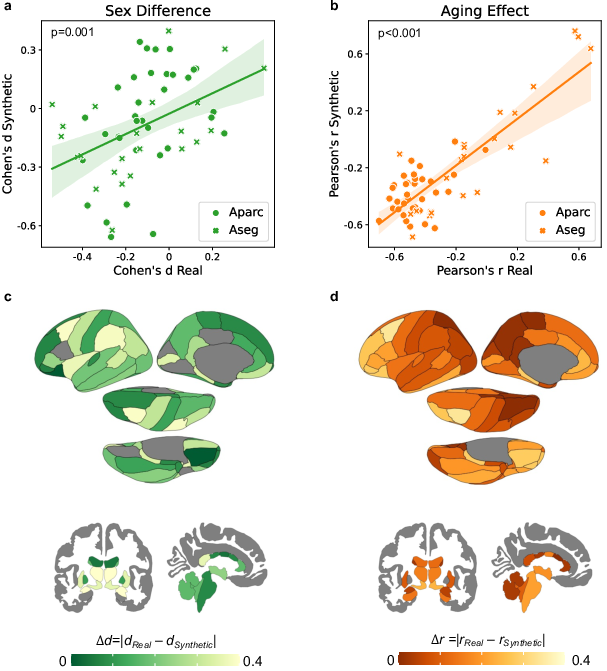
Figure 3: Sex differences (Cohen's d) and aging effects (Pearson's r) observed in synthetic MRIs significantly correlated with those measured in real MRIs.
Application in Neuroimaging Studies
A crucial application highlighted by the paper is the successful augmentation of training datasets with synthetic MRIs to improve predictive accuracies in neuroimaging studies. Specifically, the paper trained a Convolutional Neural Network (CNN) to predict age, demonstrating enhanced prediction accuracy and sensitivity to aging effects when synthetic MRIs were incorporated.
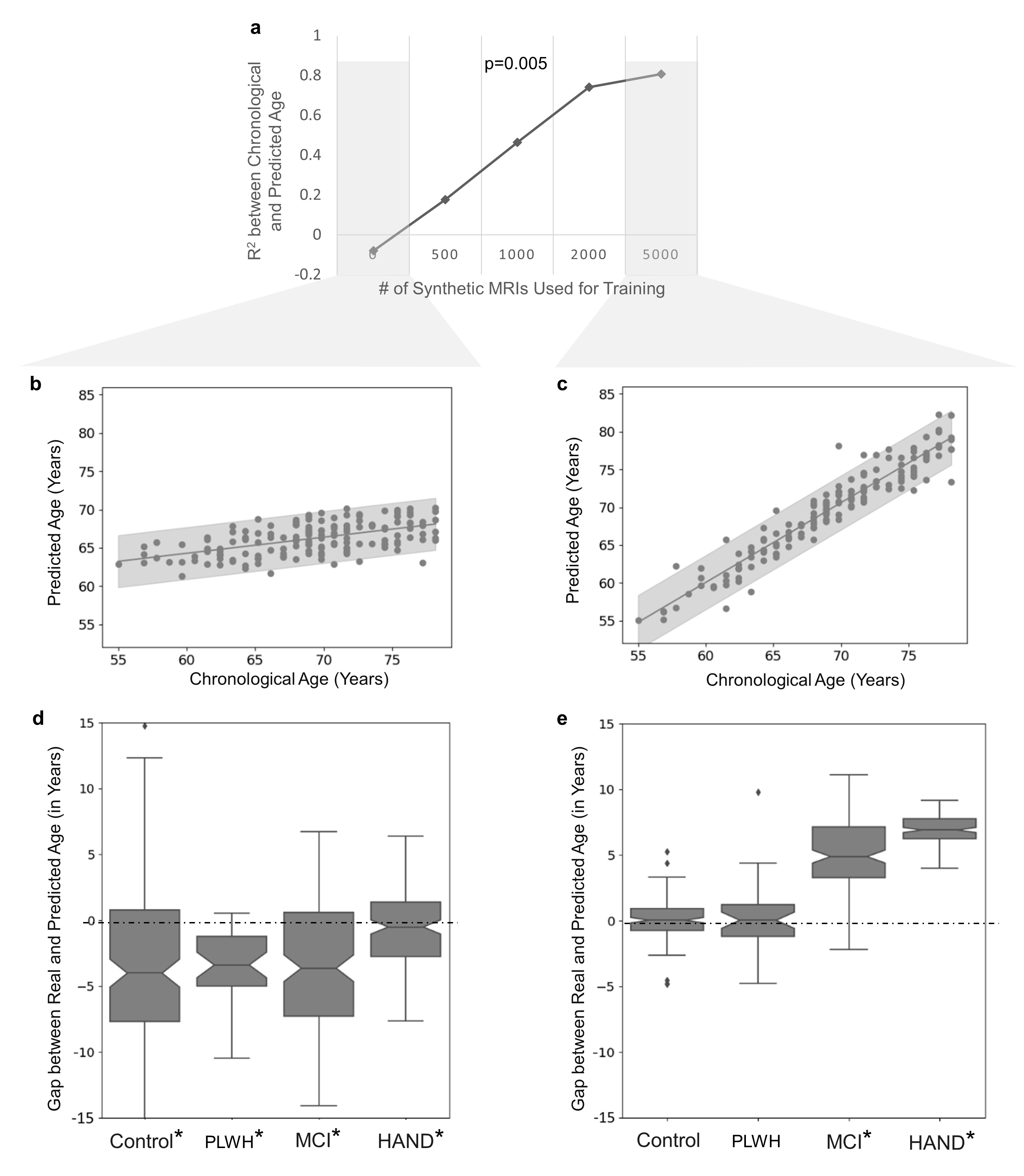
Figure 4: CNN trained with synthetic MRIs revealed accelerated aging effects in cohorts with cognitive impairment.
Conclusion
This paper demonstrates a significant advance in using generative AI for neuroimaging applications by combining high-resolution MRI synthesis with anatomical plausibility evaluation frameworks. The innovative architecture of BrainSynth not only offers a tool for generating visually accurate MRIs but also ensures anatomical credibility, bridging the gap between computational models and clinical neuroscience applications. Future research could leverage this model to further unravel biological effects and optimize training data diversity in neuroimaging studies.



