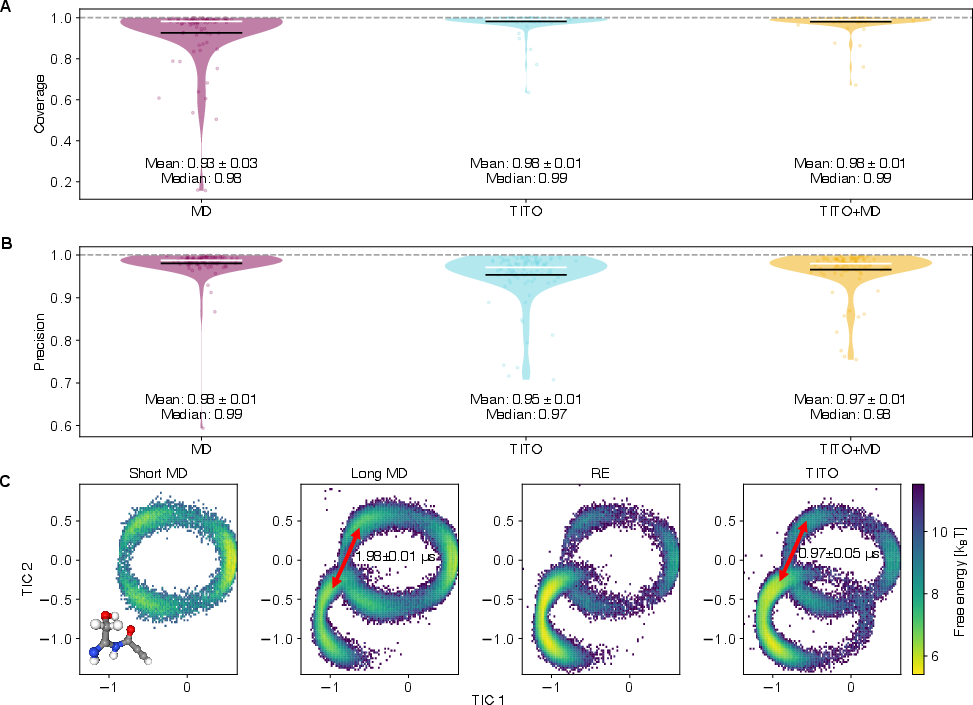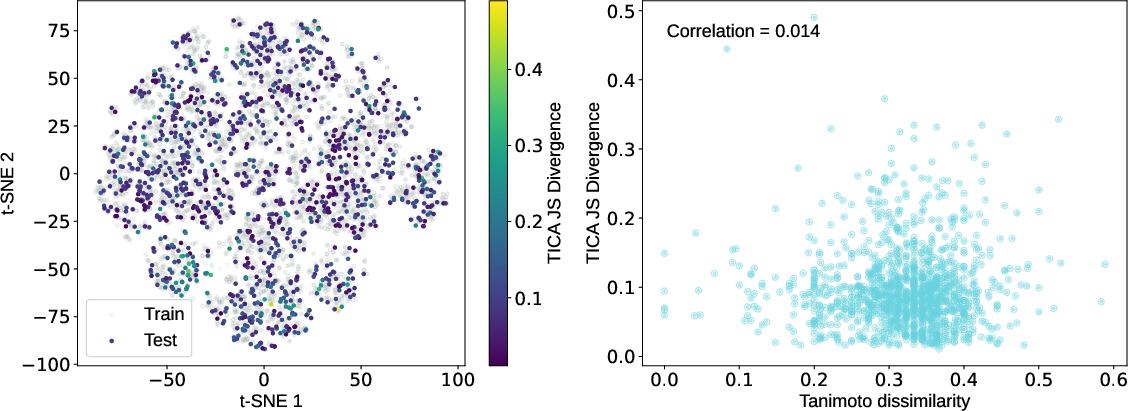Transferable Generative Models Bridge Femtosecond to Nanosecond Time-Step Molecular Dynamics (2510.07589v1)
Abstract: Understanding molecular structure, dynamics, and reactivity requires bridging processes that occur across widely separated time scales. Conventional molecular dynamics simulations provide atomistic resolution, but their femtosecond time steps limit access to the slow conformational changes and relaxation processes that govern chemical function. Here, we introduce a deep generative modeling framework that accelerates sampling of molecular dynamics by four orders of magnitude while retaining physical realism. Applied to small organic molecules and peptides, the approach enables quantitative characterization of equilibrium ensembles and dynamical relaxation processes that were previously only accessible by costly brute-force simulation. Importantly, the method generalizes across chemical composition and system size, extrapolating to peptides larger than those used for training, and captures chemically meaningful transitions on extended time scales. By expanding the accessible range of molecular motions without sacrificing atomistic detail, this approach opens new opportunities for probing conformational landscapes, thermodynamics, and kinetics in systems central to chemistry and biophysics.
Paper Prompts
Sign up for free to create and run prompts on this paper using GPT-5.
Top Community Prompts
Explain it Like I'm 14
What is this paper about?
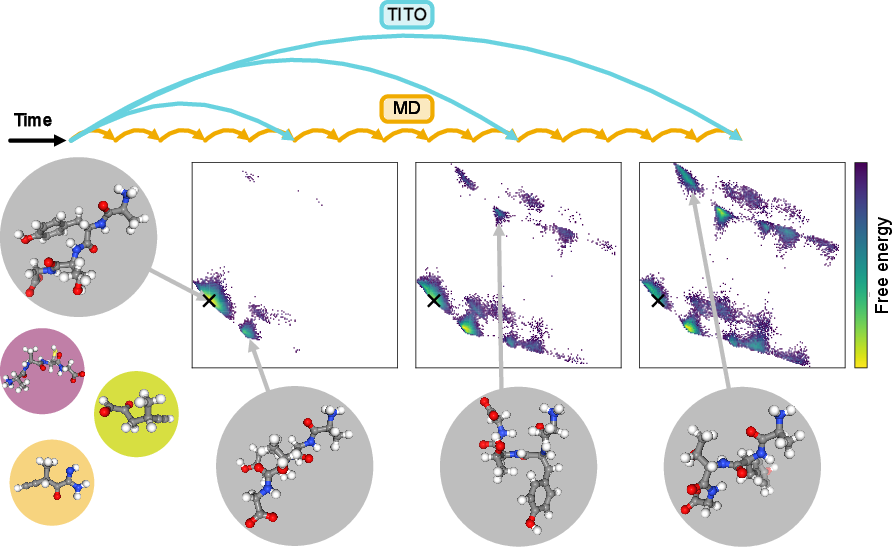
This paper introduces a new AI tool, called TITO, that can “fast‑forward” realistic computer simulations of molecules. Regular molecular dynamics (MD) simulations move atoms in tiny, super‑short steps (think: billions of frames per second). That’s great for accuracy but makes it very slow to watch big changes—like a protein folding or a drug unbinding—which can take much longer. TITO learns how molecules change over longer time jumps, so you can see important motions much faster without losing the fine, atom‑by‑atom detail.
What questions were the researchers trying to answer?
The paper asks simple but important questions:
- Can we predict how a molecule will move after a longer time step (like skipping ahead many frames) without carefully calculating every tiny step?
- Can one model work across different kinds of molecules and different time scales?
- Will the results still look like real physics—correct energies, correct shapes, and correct “how fast does this happen?” behavior?
- Can this approach run much faster than normal simulations and still be useful?
How did they do it?
To make this accessible, here’s the main idea using everyday language:
- What is molecular dynamics (MD)? MD is like a super‑detailed animated movie of atoms. You compute the forces and move the atoms a tiny bit, then repeat—millions or billions of times. Because atoms vibrate extremely fast, the “frame size” (time step) must be tiny (femtoseconds: one millionth of a billionth of a second). This makes it slow to see rare or slow events.
- The big problem: tiny time steps. Many interesting changes in chemistry (like a protein switching shape) happen over microseconds to seconds. MD can do it, but it often needs enormous computing power and a very long time.
- The new idea: TITO. Instead of calculating every tiny step, TITO learns the “leap” from one moment to a later moment directly. Think of skipping ahead in a movie while keeping the story right. TITO learns the probability of where the molecule will be after a chosen time jump—say nanoseconds—in a way that respects physics.
- How TITO learns (analogy):
- They use continuous normalizing flows (CNFs), which are like smooth recipes for reshaping one set of samples into another.
- “Flow matching” trains the neural network to move samples along the shortest path from a simple distribution to the target one.
- “Equivariant” design means the model respects symmetries—if you rotate or permute atoms sensibly, the predictions behave consistently—which is crucial for molecules.
- Training and data: They trained TITO on MD data for many small organic molecules and short peptides (chains of amino acids). Importantly, they trained TITO to handle multiple jump lengths (different lag times), so it stays consistent across time scales.
What did they find, and why is it important?
Here are the main results in plain terms:
- It stays physically realistic. TITO’s samples match the “Boltzmann distribution,” which is science‑speak for “the shapes and energies of the molecules look correct on average.” That means the model doesn’t invent impossible structures.
- It gets the timing right. TITO reproduced how fast molecules relax or switch between different shapes (metastable states), which is crucial for understanding kinetics—how quickly things happen.
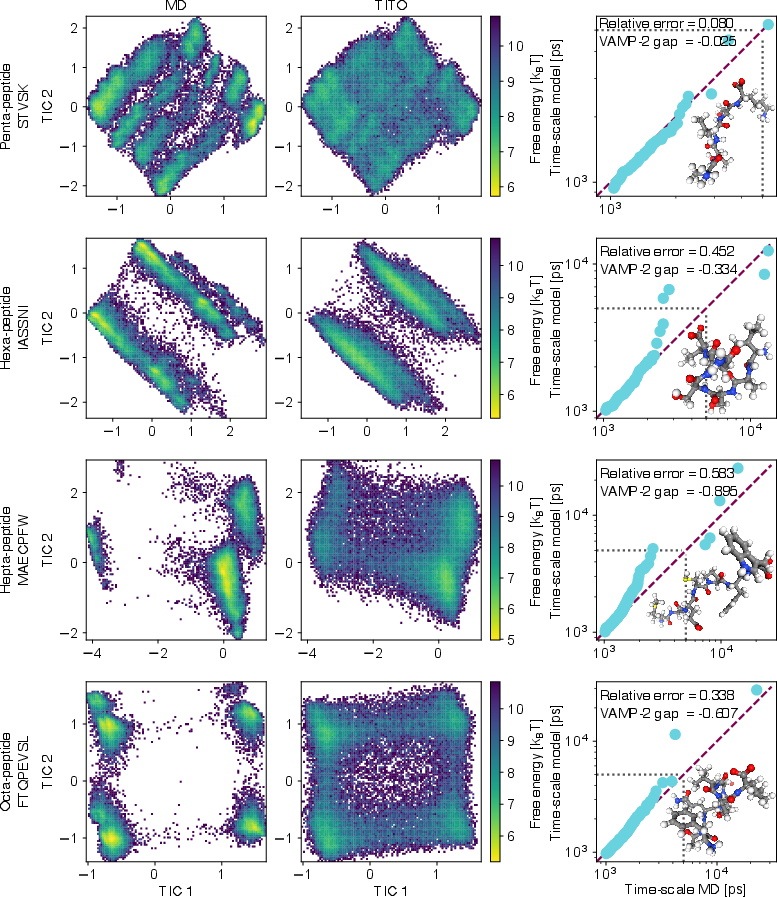
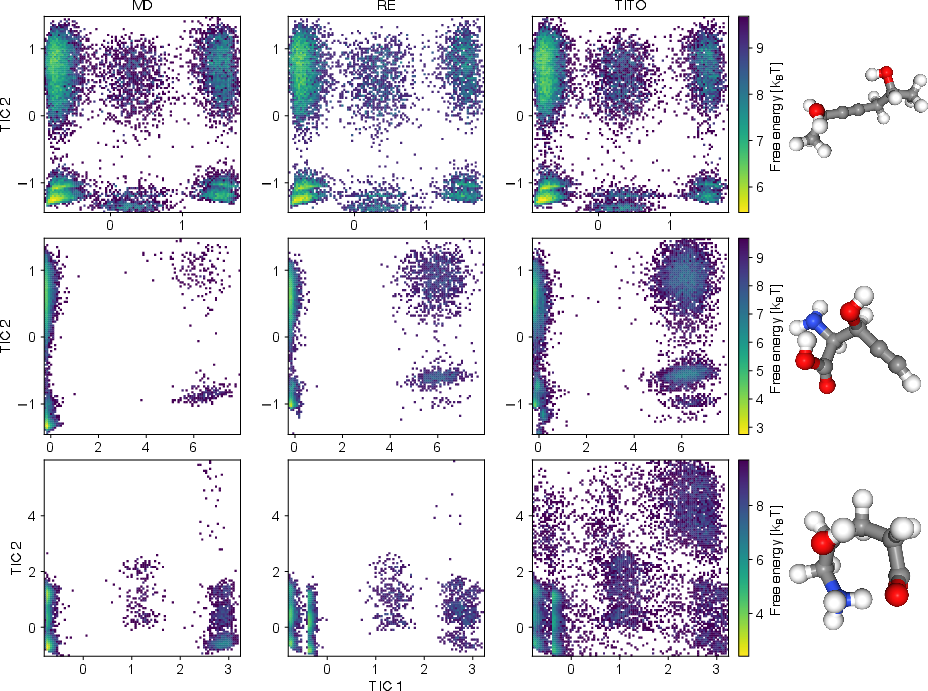
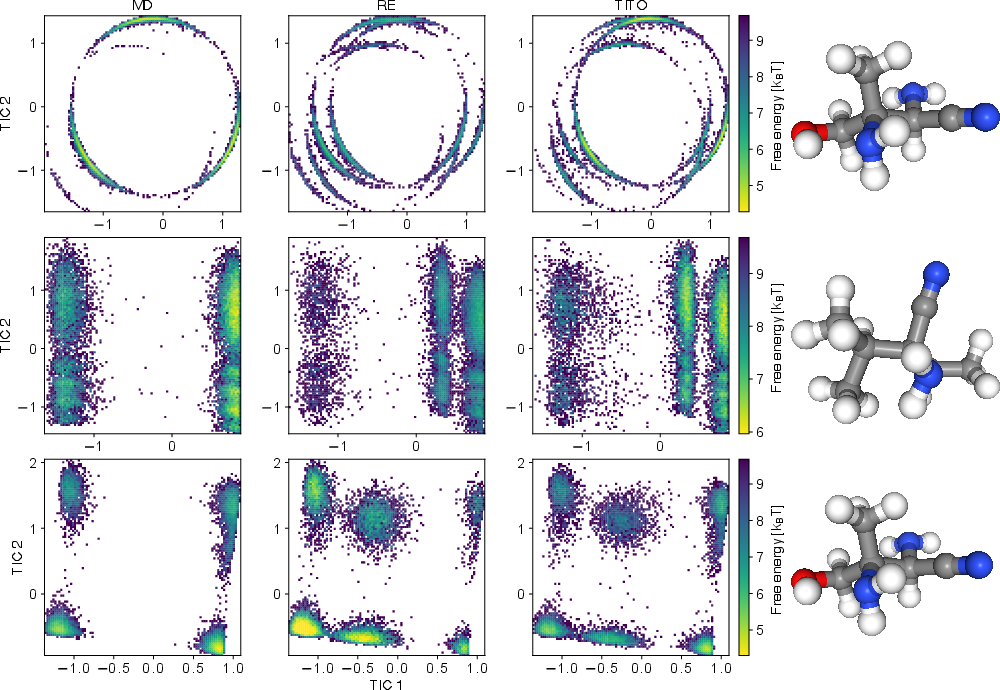
- It sometimes finds states regular MD missed. In several cases, TITO found extra stable shapes that long MD runs didn’t see. When the authors used more advanced MD methods (replica exchange), those shapes showed up, confirming TITO wasn’t making them up—it was revealing real states faster.
- It works across different molecules and time scales. TITO generalized to molecules it wasn’t trained on, and it could jump across many time steps (from femtoseconds to nanoseconds and beyond) while staying internally consistent. It even extrapolated to longer peptides than it saw in training, with a simple size scaling trick.
- It’s much faster. On the same hardware, TITO achieved around four orders of magnitude speed‑up (up to about 10 milliseconds of “physical time” per day on a single GPU), compared to typical unbiased MD simulations. That’s like compressing months of computation into hours, depending on the task.
- You can tune it. The authors showed you can adjust how much time and computing you spend to balance accuracy versus speed. For example, if you care more about overall shapes than perfect timing, you can run cheaper settings.
What does this mean going forward?
This research suggests a new way to paper molecular motion:
- It bridges the gap between very small time steps and the longer times that matter in biology and chemistry, without giving up atom‑level detail.
- It could help researchers explore protein folding, drug binding and unbinding, and other slow processes faster and more broadly.
- It may reduce the need for massive computing resources to get reliable results.
There are limitations today: TITO was tested on relatively small systems in implicit solvent (no explicit water molecules), and it currently supports one thermodynamic setting. Scaling to huge biomolecules and realistic environments will take more work. Even so, this is a promising step toward faster, physics‑aware molecular simulations powered by AI.
Knowledge Gaps
Knowledge gaps, limitations, and open questions
The paper advances transferable, long-lag generative dynamics for small molecules and peptides, but several important limitations and unresolved questions remain:
- Explicit solvent and periodic boundary conditions: How to extend TITO to explicitly solvated systems with PBC, long-range electrostatics (e.g., PME), and hydrodynamic correlations while preserving equivariance and efficiency.
- System size scaling: The current all-pairs graph and permutation/superposition alignment can scale as O(N2–N3); what architectures (sparse neighborhoods, hierarchical/coarse-grained decompositions, locality-aware attention) enable proteins with 104–105 atoms.
- Phase-space modeling: TITO conditions on positions only; extend to phase space (positions and momenta) to reproduce velocity-dependent observables (e.g., spectra, transport) and ensure kinetic-energy equipartition.
- Detailed balance and reversibility: Provide guarantees or enforceable constraints for microscopic reversibility and detailed balance of the learned transition kernel (e.g., symmetric kernels, Metropolis corrections, reversible parameterizations).
- Formal invariance to Boltzmann measure: Move from empirical JSD checks in low-dimensional projections to proofs or tight bounds that the learned operator preserves the target stationary distribution in full 3N space.
- Markovianity across lag times: Systematically quantify Chapman–Kolmogorov consistency and error versus Δt across diverse systems; define safe upper bounds on Δt where memory effects remain negligible.
- Thermodynamic transferability: Generalize across ensembles and state points (NVE/NVT/NPT; varying T, P, friction γ) and develop conditioning or reweighting strategies to move between temperatures/pressures without retraining.
- Force-field/domain transfer: Assess and improve robustness across force fields (e.g., GAFF, AMBER, CHARMM), polarizable models, and ab initio potentials; strategies for domain shift detection, adaptation, and multi-force-field training.
- Chemical coverage gaps: Evaluate on and extend to ring systems, heterocycles, charged species, metals/cofactors, PTMs, and noncanonical residues; define dataset design and active learning protocols that target missing chemistries.
- Contradictory generalization statements: Resolve the inconsistency between “no correlation with chemical similarity” (Results) and “generalization depends on chemical similarity” (Discussion); clarify metrics, datasets, and scope.
- Kinetics calibration and absolute rates: Establish procedures to calibrate absolute timescales (not only implied timescales) against MD/experiment, including uncertainty quantification and potential re-scaling across Δt choices.
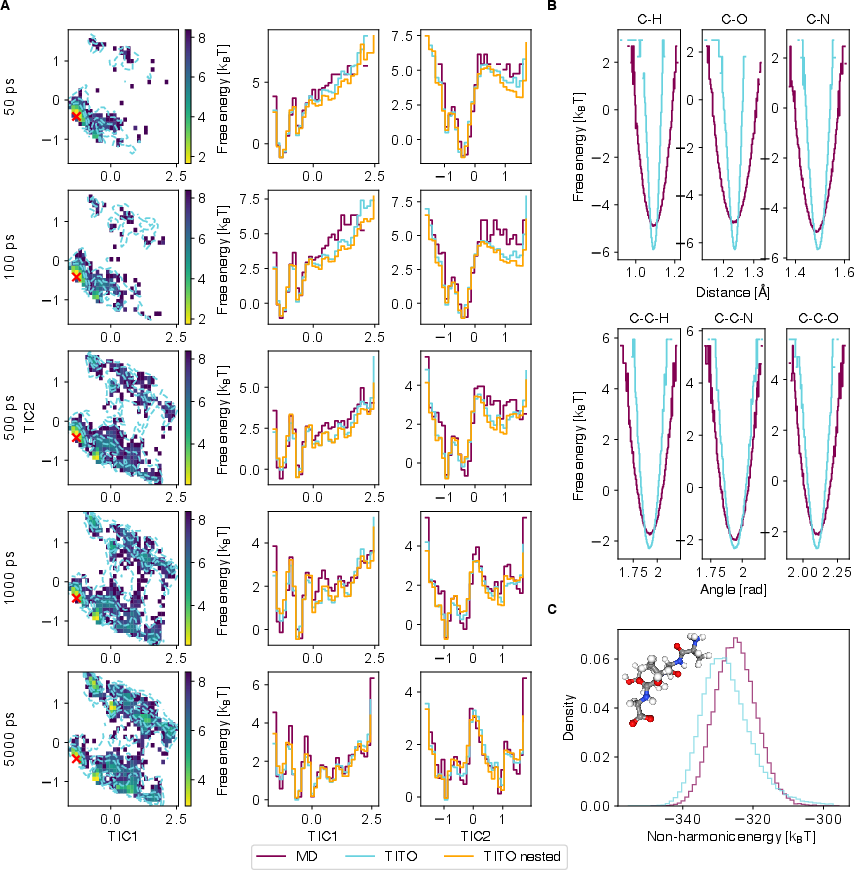
- Fast-mode fidelity: Address systematic underestimation of fast vibrational variances and potential-energy drift (especially for larger peptides) via multi-scale losses, physics-informed regularization, or local-mode constraints.
- Hybrid TITO–MD schemes: Design principled hybrid algorithms (e.g., TITO proposals with short MD relaxations and Metropolis acceptance) with proofs of stationarity, quantified bias, and practical schedules.
- Data efficiency and compute costs: Quantify training data requirements, learning curves, and training/inference cost breakdowns; develop active learning loops that target dynamical motifs most informative for operator learning.
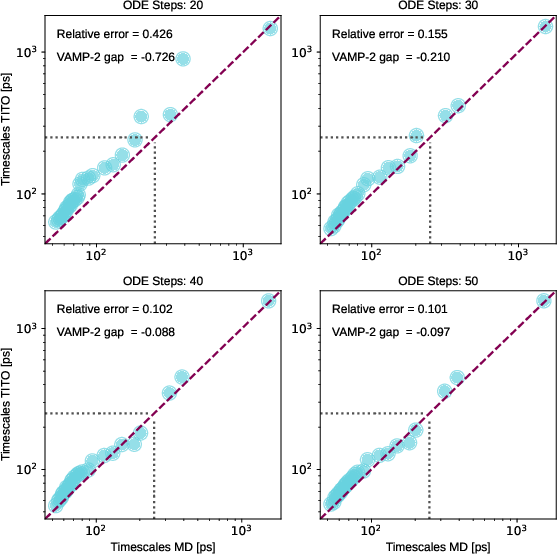
- ODE solver and sampler efficiency: Explore solver tolerance/step-size trade-offs, distillation to few- or single-step samplers, stochastic flow variants, and specialized integrators that reduce compute while preserving accuracy.
- Conditioning on system size/sequence: Replace heuristic Flory scaling of the base distribution with learned, sequence- and size-conditional base distributions or adaptive normalization to stabilize extrapolation.
- Non-equilibrium and driven processes: Extend to time-dependent or non-stationary dynamics (external fields, pulling, chemical gradients) and compute response functions/transport coefficients reliably.
- Validation breadth and realism: Benchmark on larger, realistic biomolecular tasks (folding, binding, allostery) in explicit solvent with experimental comparisons (e.g., FRET/NMR rates) and report absolute kinetic observables and mechanisms.
- Transition-path fidelity: Validate path-ensemble properties (committor distributions, transition-path times, mechanism heterogeneity) rather than only two-time statistics.
- Reactivity and topology changes: Extend to bond breaking/forming, proton transfer, tautomerization, and changes in bond orders; handle variable molecular graphs and changing edge features during dynamics.
- Constraints and holonomic models: Incorporate and test rigid-bond/angle constraints (e.g., SHAKE) and ensure consistency of constrained dynamics under long-lag transitions.
- PBC-aware equivariance: Develop E(3)-equivariant modeling on periodic tori (minimum image) to avoid artifacts at cell boundaries.
- Uncertainty quantification and “hallucinated” states: Provide calibrated uncertainties for predicted states/timescales, diagnostics to detect spurious basins, and protocols to vet novel states (e.g., automated RE/umbrella validations).
- Failure-mode analysis: Identify molecular or dynamical descriptors predictive of TITO errors, guiding dataset curation and active sampling; reconcile cases where TITO predicts dynamics slower than theoretical bounds.
- Comparisons to operator-learning baselines: Systematically compare accuracy/throughput to VAMPnets/Koopman models, SDE/score-based simulators, and path-sampling methods, including ablations on architecture and losses.
- Differentiability and inverse problems: Investigate whether TITO enables differentiable dependence on thermodynamic/force-field parameters for inverse design, model calibration, or enhanced sampling control.
Practical Applications
Immediate Applications
Below are actionable use cases that can be deployed now, grounded in the paper’s findings on TITO’s ability to generate Boltzmann-consistent ensembles and relaxation dynamics at multi-nanosecond to microsecond lag times with four orders-of-magnitude acceleration.
- Accelerated ensemble generation for small molecules and short peptides
- Sector: healthcare (pharma/biotech), cheminformatics, software
- What: Generate thermodynamically consistent conformational ensembles and kinetics (e.g., implied timescales) for small molecules and tetra-peptides in implicit solvent.
- Potential tools/products/workflows: “Kinetics-aware conformer generator” (TITO + RDKit/OpenMM); TITO-driven conformer libraries for docking/scoring; API to sample at user-selected lag times to match assay windows.
- Assumptions/dependencies: Trained on implicit solvent, GAFF force-field; good transfer within similar chemical/size regimes; solver-step calibration affects kinetic accuracy; fast-mode variance is slightly underestimated.
- Discovery of hidden metastable states missed by conventional MD
- Sector: healthcare (medicinal chemistry), materials, academia
- What: Use TITO to uncover metastable basins and slow transitions beyond practical MD limits; validate candidates with replica-exchange (RE) MD or short unbiased MD seeded from TITO samples.
- Potential tools/products/workflows: “TITO→RE MD validation loop”; metastable-basin mining for binding modes, conformational gating, isomerization.
- Assumptions/dependencies: Some TITO-unique states require independent validation; underlying reference force-field quality bounds realism.
- Low-cost kinetic analysis and MSM construction
- Sector: academia, healthcare
- What: Build Markov State Models (MSMs) and estimate implied timescales and relaxation transients using TITO trajectories.
- Potential tools/products/workflows: Integration with PyEMMA/MDAnalysis; VAMP-based diagnostics (VAMP-2, VAMP-gap); Chapman–Kolmogorov consistency checks using nested vs single-step sampling.
- Assumptions/dependencies: Model trained on relevant chemistries; lag-time consistency hinges on ODE solver step counts; NVT implicit solvent.
- Alignment of simulations to experimental timescales
- Sector: academia, healthcare
- What: Choose Δt to match observation windows (NMR relaxation, single-molecule FRET), enabling hypothesis generation and experiment design.
- Potential tools/products/workflows: Protocols to co-design lag times with experimental readouts; rapid scanning of kinetic regimes.
- Assumptions/dependencies: NVT implicit solvent; temperature/pressure fixed; mapping to experimental buffers may require correction.
- MD initialization and pre-equilibration
- Sector: software, healthcare, materials
- What: Use TITO to pre-sample diverse, near-physical configurations to initialize short MD runs, reducing time to reach relevant basins.
- Potential tools/products/workflows: “TITO-initializer” plugins for OpenMM/GROMACS; hybrid TITO + short MD equilibration.
- Assumptions/dependencies: Best for small molecules/short peptides; periodic boundary conditions not supported; slight energy-variance drift on larger systems.
- Education and interactive visualization
- Sector: education, daily life (STEM outreach)
- What: Web-based demos of realistic molecular motions and relaxation transients at experimentally relevant timescales, using pre-trained TITO models.
- Potential tools/products/workflows: Browser-based molecule explorer; classroom modules demonstrating Boltzmann distributions and metastability.
- Assumptions/dependencies: Pre-trained models limited to implicit solvent and small systems; educational, not for quantitative research.
- Compute efficiency and sustainability
- Sector: policy, industry (R&D ops), HPC centers
- What: Replace portions of brute-force MD with TITO to achieve ~10 ms/day physical simulation time per A100 GPU (vs μs-scale for MD), cutting energy use and cost.
- Potential tools/products/workflows: Internal guidelines to deploy TITO for ensemble generation and initial kinetic characterization; reporting protocols using JSD/VAMP metrics.
- Assumptions/dependencies: Validation requirements; governance for model use in regulated contexts; careful calibration of accuracy vs throughput.
- Data augmentation for ML potentials and coarse-grained models
- Sector: academia, software
- What: Generate diverse ensembles to enrich training datasets for ML force fields/coarse-grained models, especially in under-sampled basins.
- Potential tools/products/workflows: TITO-augmented training sets; active learning loops to target dynamical motifs.
- Assumptions/dependencies: Consistency with downstream target potentials; risk of propagating bias from source MD training data.
- Standardized acceptance metrics for simulation coverage/kinetics
- Sector: industry, academia
- What: Adopt JSD, coverage/precision, and VAMP-gap as routine acceptance criteria in simulation pipelines.
- Potential tools/products/workflows: Benchmark dashboards integrating TITO and MD outputs; automated alerts for kinetic mismatches.
- Assumptions/dependencies: Availability of suitable reference trajectories; thresholds tuned to application-specific tolerances.
Long-Term Applications
Below are use cases that require further research, scaling, or development (e.g., explicit solvent, periodic boundary conditions, larger systems, thermodynamic transferability).
- Protein–ligand binding and unbinding kinetics at scale
- Sector: healthcare (drug discovery)
- What: Extend TITO to explicit solvent and periodic boundary conditions for proteins/complexes to estimate binding/unbinding rates and mechanistic pathways.
- Potential tools/products/workflows: “TITO-XL” for biomolecular complexes; binding kinetics dashboards; triage of candidates by rate and mechanism.
- Assumptions/dependencies: New neural architectures/hierarchical strategies; diverse training datasets; robust validation against experiments and long MD.
- Transport and relaxation in complex materials (electrolytes, polymers, ionic liquids)
- Sector: energy, materials
- What: Predict diffusion, viscosity, and relaxation times across extended timescales in solvated or bulk materials.
- Potential tools/products/workflows: PBC-enabled TITO for condensed-phase systems; property calculators linked to materials design workflows.
- Assumptions/dependencies: Explicit solvent support; thermodynamic conditioning; scalable training with large system sizes.
- Thermodynamic transferability across ensembles (T, P)
- Sector: academia, industry
- What: Condition TITO on temperature/pressure to simulate across thermodynamic states; interpolate kinetics/thermodynamics across conditions.
- Potential tools/products/workflows: Thermodynamic interpolation modules; multi-ensemble training regimes; experiment–simulation alignment tools.
- Assumptions/dependencies: Training at multiple T/P; extensions to NPT; careful validation of ensemble consistency.
- Hybrid multi-resolution simulation frameworks
- Sector: software, academia
- What: Interleave long-lag TITO propagation with short MD equilibration to control local errors and maintain stability in large systems.
- Potential tools/products/workflows: Plugins for hybrid TITO↔MD workflows; adaptive schedules based on error monitors (e.g., energy drift).
- Assumptions/dependencies: Error control strategies; detailed balance considerations; robust diagnostics for convergence.
- Generative path-sampling for rare events (hyperdynamics-style)
- Sector: academia (theoretical/computational chemistry)
- What: Use TITO as a proposal engine for rare-event sampling (e.g., conformational switches, reactions with reactive potentials).
- Potential tools/products/workflows: TITO-guided transition path sampling; coupling to enhanced sampling and reweighting schemes.
- Assumptions/dependencies: Reactive force fields and appropriate datasets; kinetic fidelity under reweighting; rigorous validation.
- Inverse design of molecular dynamics (optimize for target kinetics)
- Sector: healthcare, materials
- What: Close the loop between generative dynamics and design to optimize molecules for specific relaxation times, metastability, or gating mechanisms.
- Potential tools/products/workflows: Active learning platforms where kinetic objectives guide candidate generation and evaluation; gradient-free or surrogate-based optimization.
- Assumptions/dependencies: Reliable structure–dynamics mapping; generalization beyond training distribution; multi-objective trade-offs (kinetics vs thermodynamics).
- Citizen science 2.0 and HPC sustainability policy
- Sector: policy, education
- What: Transition from brute-force distributed MD to validated generative surrogates to lower carbon footprints while delivering comparable scientific outputs.
- Potential tools/products/workflows: Governance frameworks for model validation; public dashboards; audit trails for surrogate-based science.
- Assumptions/dependencies: Community trust; reproducibility standards; clear protocols for where surrogates replace (or complement) MD.
- Cloud “MD Surrogate as a Service”
- Sector: software, industry
- What: Offer TITO models via API to generate ensembles/kinetics on demand with controllable Δt and accuracy–throughput budgets.
- Potential tools/products/workflows: Managed services with job queuing, auto-calibration of solver steps, and standardized output (ensembles, timescales, diagnostics).
- Assumptions/dependencies: Secure handling of proprietary molecules; SLA around accuracy; support for custom training.
- Community dynamical benchmarks and curated datasets
- Sector: policy, academia
- What: Establish benchmarks focused on dynamical motifs and energy landscapes (beyond mere chemical coverage) to drive generalization in generative dynamics.
- Potential tools/products/workflows: Open datasets and leaderboards; standardized metrics (JSD/VAMP); funding programs prioritizing mechanistic diversity.
- Assumptions/dependencies: Broad buy-in; data sharing across sectors; consistent evaluation protocols.
- Reaction mechanism exploration
- Sector: chemistry, catalysis
- What: Extend TITO to reactive potentials to explore long-time reaction pathways, barrier crossings, and catalyst conformational dynamics.
- Potential tools/products/workflows: TITO-guided reaction discovery; coupling with quantum chemistry for barrier validation.
- Assumptions/dependencies: Reactive FF/ML potentials; energy conservation and reactivity modeling; integration with electronic-structure calculations.
Glossary
- Allosteric regulation: Regulation of protein function via conformational changes at sites distinct from the active site, often modulating activity. "the conformational transitions underlying allosteric regulation"
- Boltzmann distribution: The equilibrium probability distribution over states proportional to exp(−βU(x)), where U is potential energy and β is inverse temperature. "the Boltzmann distribution, "
- Boltzmann Emulators: Generative models that emulate Boltzmann sampling while trading off quantitative agreement with MD for efficiency and scalability. "Boltzmann Emulators which sacrifice quantitative alignment with MD to boost efficiency and scaling"
- Boltzmann equilibrium: The condition where sampled configurations follow the Boltzmann distribution at a given thermodynamic state. "preserves key statistical properties such as Boltzmann equilibrium, Markovianity, and relaxation dynamics"
- Boltzmann Generators: Deep generative models designed to directly sample equilibrium states from the Boltzmann distribution. "Boltzmann Generators which aim to quantitatively sample the independent equilibrium samples from the Boltzmann distribution"
- Chapman–Kolmogorov equation: The consistency relation for Markov processes stating that multi-step transitions equal compositions of shorter-step transitions. "satisfies the Chapman–Kolmogorov equation and thus encodes genuinely Markovian dynamics"
- Coarse-graining: Reducing the degrees of freedom of a system to model effective dynamics at a larger scale. "hierarchical strategies such as coarse-graining"
- Collective variables (CVs): Low-dimensional descriptors that capture progress along complex processes to guide enhanced sampling. "Biasing methods rely on the definition of collective variables (CVs), that capture the progress of a process of interest"
- Continuous normalizing flow (CNF): A generative model defined by a neural ODE that transports a base distribution to match target data. "A CNF consists of an ordinary differential equation (ODE) and an easy-to-sample `base distribution,' , such as a Gaussian"
- Equipartition: The equilibrium principle that energy is equally partitioned among quadratic degrees of freedom. "suggesting approximate energy conservation and equipartition."
- Equivariant optimal transport flow matching: A flow-matching training method that exploits symmetries (e.g., rotations, permutations) to align transport velocities. "TITO uses equivariant optimal transport flow matching to parameterize the transition probability."
- Flory’s scaling law: A polymer physics relation predicting how radius of gyration scales with chain length, here . "Flory’s scaling law for the radius of gyration of random polymers, "
- Fokker–Planck equation: The partial differential equation governing the time evolution of probability densities under stochastic dynamics (e.g., Langevin). "the Green’s function of the Fokker-Planck equation"
- Free energy: A thermodynamic quantity (often −kBT log probability) that characterizes the stability and likelihood of states. "Free energy profiles of bond distances (top) and angles (bottom)."
- GAFF force field: The General AMBER Force Field used for simulating small organic molecules. "using the GAFF force field"
- Green’s function: The fundamental solution describing transition probabilities for a stochastic or differential operator. "approximates the Green’s function of the Fokker-Planck equation"
- Implicit solvent representations: Modeling solvent effects without explicit solvent molecules, typically via continuum approximations. "At present, TITO is restricted to implicit solvent representations"
- Implied timescales: Characteristic relaxation times inferred from the eigenvalues/singular values of an estimated transfer/Koopman operator at a chosen lag. "The eigenvalues of the estimated transfer operator were used to compute implied timescales"
- Invariant measure (stationary distribution): A probability distribution that remains unchanged under the application of the system’s transfer operator. "is the invariant measure (i.e., stationary distribution) of the transfer operator implicitly learned by TITO"
- Jensen–Shannon divergence (JSD): A bounded, symmetric divergence measuring similarity between two probability distributions. "using the Jensen–Shannon divergence (JSD)"
- Koopman operator: A linear operator acting on observables to propagate them under nonlinear dynamics, used to analyze slow modes. "we constructed Koopman operator models from long unbiased MD and from TITO trajectories."
- Langevin equation: A stochastic differential equation describing particle dynamics with deterministic forces, friction, and random noise. "numerical integration of the Langevin equation"
- Linear sum assignment problem: An optimization problem to find a minimum-cost matching between two sets (often solved via Hungarian algorithm). "solving a linear sum assignment problem"
- Markov state models (MSMs): Statistical models that represent dynamics as transitions between metastable states with Markovian kinetics. "statistical frameworks such as Markov state models (MSMs)"
- Markovianity: The property that future evolution depends only on the current state, not on past history. "preserves key statistical properties such as Boltzmann equilibrium, Markovianity, and relaxation dynamics"
- Metastable states: Long-lived states separated by barriers, leading to rare transitions and slow kinetics. "rare transitions between metastable states"
- Nested sampling: Composing long-lag trajectory generation from multiple shorter steps to check consistency across time resolutions. "Nested samples are generated with 5 steps."
- NVT ensemble: A thermodynamic ensemble with fixed number of particles (N), volume (V), and temperature (T). "we here target MD in the NVT ensemble"
- Ordinary differential equation (ODE): An equation governing continuous-time change; here the dynamical backbone of CNFs. "A CNF consists of an ordinary differential equation (ODE) and an easy-to-sample `base distribution,' , such as a Gaussian"
- Periodic boundary conditions: Simulation conditions where the system is replicated in space to mimic bulk behavior and avoid edge effects. "periodic boundary conditions—essential for realistic modeling of solvated systems—are not yet supported."
- Procrustes problem: Finding the optimal rotation/translation (and possibly scaling) to superimpose two point sets. "through solving a Procrustes problem"
- Radius of gyration: A measure of the spatial extent or compactness of a molecule/polymer. "radius of gyration of random polymers"
- Replica exchange (RE) MD: An enhanced sampling technique where simulations at different temperatures exchange configurations to cross barriers. "replica exchange (RE) MD simulations"
- SE3-ITO architecture: An SE(3)-equivariant neural architecture variant used in TITO’s velocity field model. "we use a modified SE3-ITO architecture"
- Thermodynamic transferability: The ability of a model to generalize across different temperatures/pressures while preserving thermodynamic behavior. "Extending thermodynamic transferability, including across temperatures or pressures"
- Time-lagged independent component analysis (TICA): A method to identify slow collective modes by analyzing time-lagged correlations. "Projection onto the first two TICA components"
- Transfer operator: The operator that advances probability distributions (or densities) forward in time under the dynamics. "the transfer operator implicitly learned by TITO"
- Transition probability distribution: The conditional distribution of future states given the current state at a chosen lag time. "transition probability distribution "
- Variational Approach to Markov Processes (VAMP): A framework to learn and assess dynamical models via variational principles on singular functions/values. "VAMP (Variational Approach to Markov Processes)"
- VAMP-2 score: A metric equal to the squared Frobenius norm of the singular value spectrum, summarizing captured slow modes. "The VAMP-2 score is the squared Frobenius norm of the singular value spectrum"
- VAMP-gap: The difference in VAMP-2 scores between two models, indicating relative capture of slow dynamics. "We define the VAMP-gap as the difference in VAMP2-scores between TITO and the MD"
Collections
Sign up for free to add this paper to one or more collections.