- The paper introduces a novel memristor-driven spike encoding method using piezo-MEMS cantilevers and VO₂ nanogap oscillators for cochlear implants.
- It demonstrates linear spike rate encoding of vibroacoustic stimuli with sharp frequency selectivity and flexible waveform generation (unipolar and biphasic).
- Experimental results validate low power consumption, high reproducibility, and scalability for fully implantable, energy-efficient auditory prostheses.
Memristor-Driven Spike Encoding for Fully Implantable Cochlear Implants
Introduction and Motivation
This work addresses the critical challenge of developing energy-efficient, fully implantable cochlear implants (FICIs) by introducing a bio-inspired auditory sensing system that leverages piezo-MEMS cantilevers and VO2 nanogap memristor-based oscillators. The motivation stems from the limitations of conventional cochlear implants, which rely on external components and power-hungry digital signal processing, leading to issues such as social stigmatization, maintenance difficulties, and limited battery life. The proposed system aims to overcome these barriers by mimicking the natural auditory pathway, enabling direct, low-power, frequency-selective spike encoding suitable for direct neural stimulation.
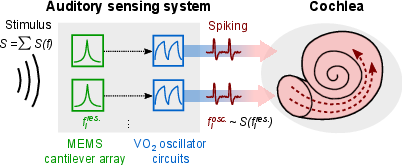
Figure 1: Conceptual overview of the bio-inspired auditory sensing system, showing frequency-selective piezo-MEMS cantilevers interfaced with VO2 memristor-based oscillators for spike encoding.
System Architecture and Component Design
Piezo-MEMS Cantilever Array
The front-end of the system consists of an array of spiral-shaped piezo-MEMS cantilevers, each engineered to have a distinct resonance frequency within the 200–700 Hz range, corresponding to the fundamental frequencies of human speech. The cantilevers are fabricated from ScAlN and optimized for high Q-factor operation, ensuring sharp frequency selectivity and high sensitivity to nanometer-scale vibrations, as encountered in the middle ear.
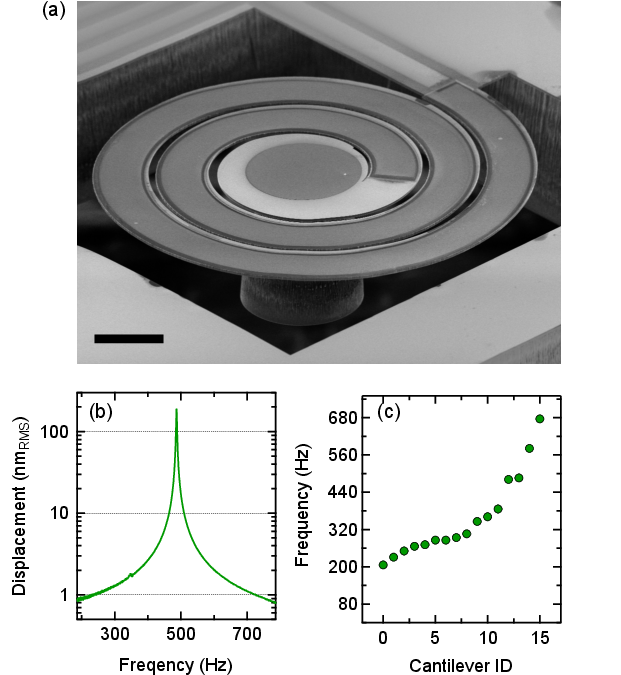
Figure 3: Characterization of piezo-MEMS cantilevers, demonstrating high Q-factor and tunable resonance frequencies across the speech-relevant domain.
VO2 Nanogap Memristor-Based Oscillators
The core innovation lies in the use of volatile VO2 memristors as the active element in relaxation oscillators. These memristors exhibit Mott-type insulator-to-metal (IMT) and metal-to-insulator (MIT) transitions, enabling the generation of action potential-like spiking waveforms. The planar nanogap geometry (30–60 nm) ensures high electric field concentration and efficient switching, with resistance states spanning RLRS⪅1 kΩ and RHRS≈30 kΩ.
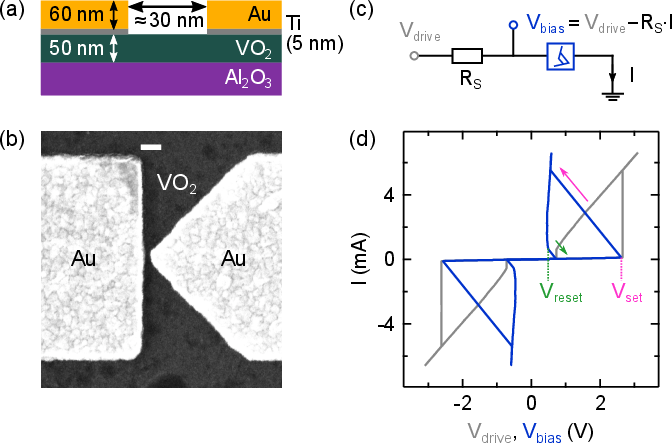
Figure 4: Fabrication and electrical characterization of VO2 nanogap memristors, highlighting the sharp IMT/MIT transitions and suitability for spiking oscillator circuits.
The oscillator circuit comprises a series RC network with the memristor, where the input DC voltage (derived from the rectified MEMS output) modulates the spiking frequency. The circuit parameters (R, C, and Vin) are tuned to produce biologically relevant spike rates (100 Hz–1 kHz), with the memristor's threshold voltages (Vset, Vreset) dictating the oscillation regime.
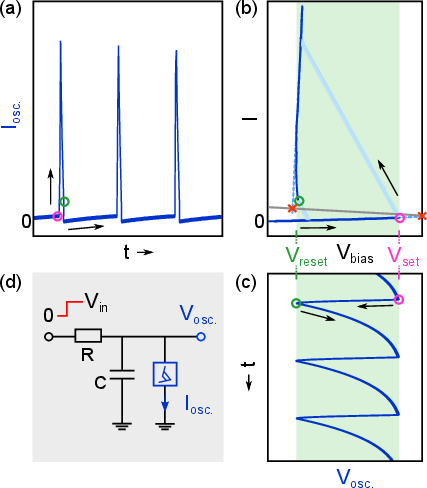
Figure 5: Relaxation oscillator operation, showing current and voltage waveforms, and the load-line analysis of the memristor's I–V characteristics.
Signal Processing and Spike Encoding
Frequency-Selective Sensing and Rate Encoding
Each cantilever senses vibroacoustic stimuli at its resonance frequency, generating an AC voltage proportional to the vibration amplitude. This signal is amplified, rectified, and smoothed to produce a DC voltage that serves as the input to the memristor oscillator. The resulting output is a train of current spikes, with the spike rate linearly encoding the amplitude of the incoming stimulus—a direct analog to rate coding in the auditory nerve.
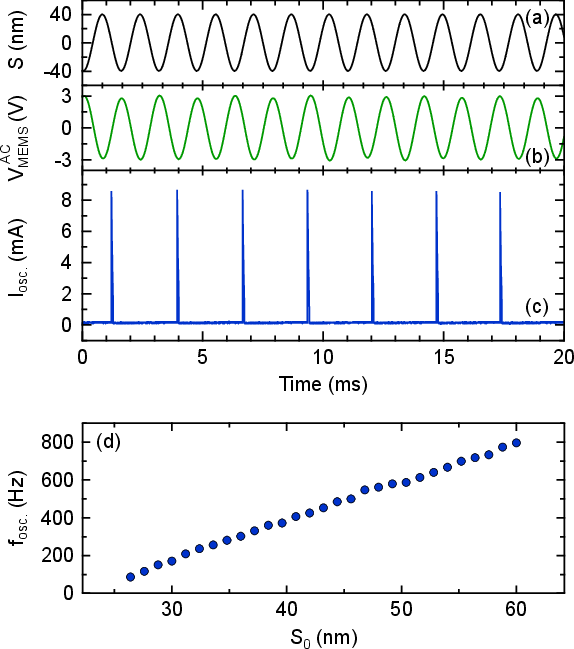
Figure 7: Electromechanical experiment results showing the transformation of controlled sinusoidal stimuli into spike trains, with spike rate proportional to stimulus amplitude.
The system demonstrates robust rate encoding across biologically realistic displacement amplitudes (10–60 nm), corresponding to 82–92 dB SPL at the umbo. The spike rate can be precisely controlled via the input voltage, and the system operates reliably within the auditory nerve's physiological firing range.
A critical requirement for neural stimulation is the use of biphasic (charge-balanced) waveforms to prevent electrode degradation and tissue damage. The native output of the oscillator is unipolar; however, by introducing a parallel LR network at the output, the system can generate biphasic spikes with minimal circuit overhead. This approach yields ramped biphasic pulses, which have been shown to be as effective as rectangular pulses for neural activation and may offer improved energy efficiency.
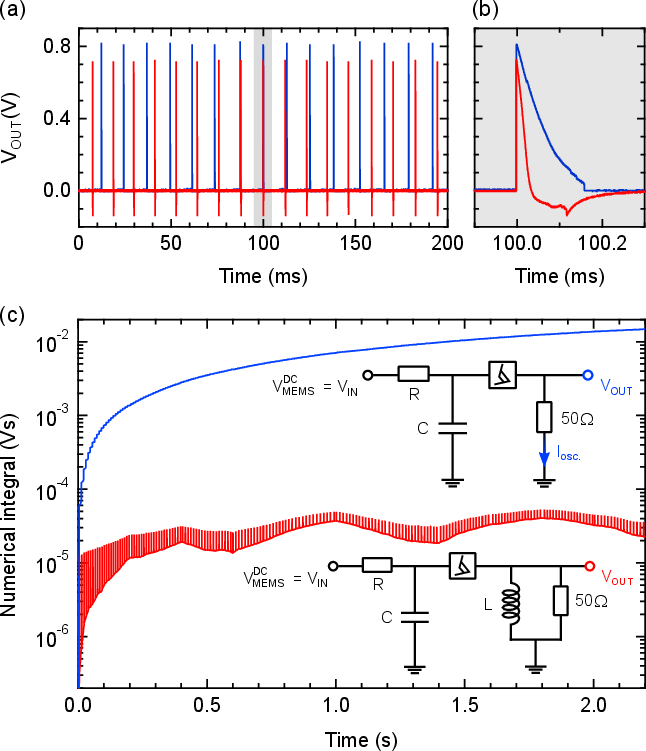
Figure 2: Comparison of unipolar and biphasic output waveforms, demonstrating effective charge balancing via circuit modification.
Implementation Considerations
Fabrication and Integration
- MEMS Cantilevers: Fabricated using standard microfabrication techniques, with spiral geometry for tunable resonance. Integration with custom PCBs and ribbon cables allows for scalable array assembly.
- VO2 Memristors: Grown via pulsed laser deposition on sapphire substrates, with electron-beam lithography for nanogap electrode definition. The planar design facilitates monolithic integration with MEMS arrays.
- Circuit Integration: The system is amenable to miniaturization and monolithic integration, supporting the stringent size and power constraints of FICIs.
Power Consumption and Scalability
The system eliminates the need for FFT-based digital processing, leveraging analog frequency selectivity and direct spike encoding. The memristor-based oscillators operate at sub-microwatt power levels, and the entire front-end can be powered by a small, non-rechargeable battery, addressing a major limitation of current FICI designs.
Biocompatibility and Implantation
All components are compatible with established biocompatible encapsulation techniques. The MEMS array can be positioned near the ossicular chain, and the output can be interfaced directly with multielectrode arrays for auditory nerve stimulation.
- Spike Rate Linearity: The output spike rate exhibits a linear dependence on stimulus amplitude over the tested range, with high reproducibility.
- Frequency Selectivity: The MEMS array provides sharp frequency discrimination, supporting multi-channel operation analogous to the tonotopic organization of the cochlea.
- Waveform Flexibility: The system supports both unipolar and biphasic output modes, with tunable pulse shapes via simple circuit modifications.
- Biological Relevance: The system operates within the displacement and spike rate regimes observed in the human auditory system.
Implications and Future Directions
This work demonstrates the feasibility of a fully analog, bio-inspired front-end for cochlear implants, leveraging memristor-based neuromorphic circuits for direct spike encoding. The approach offers significant advantages in terms of power efficiency, miniaturization, and biomimetic information processing. The direct analog-to-spike conversion paradigm could be extended to other sensory prostheses and neuromorphic interfaces.
Future research directions include:
- Scaling to Multi-Channel Arrays: Integration of larger MEMS-memristor arrays to cover the full auditory spectrum and enable complex sound encoding.
- Closed-Loop Neural Interfaces: Incorporation of feedback from neural recordings to adaptively tune spike encoding parameters.
- Long-Term Biocompatibility Studies: In vivo validation of chronic stability and safety of the integrated system.
- Advanced Spike Coding Schemes: Exploration of temporal and population coding strategies for richer auditory information transmission.
Conclusion
The presented memristor-driven spike encoding system represents a significant step toward fully implantable, energy-efficient cochlear implants. By combining frequency-selective MEMS sensors with VO2 memristor-based oscillators, the system achieves direct, biomimetic spike encoding of acoustic stimuli, with demonstrated capability for rate coding and biphasic waveform generation. The architecture is well-suited for monolithic integration and low-power operation, addressing key challenges in the development of next-generation sensory prostheses.







