- The paper introduces a non-genetic cell surface engineering approach for programmable, biocompatible Bio-Nano Things in IoBNT.
- It demonstrates both covalent and non-covalent strategies to functionalize cell membranes using DNA nanostructures, nanoparticles, and polymers.
- The approach enables dynamic modulation of cellular sensing, communication, and mobility, paving the way for advanced diagnostics and bio-cyber interfacing.
Non-Genetic Cell Surface Engineering for Programmable Living Bio-Nano Things in IoBNT
Introduction and Motivation
The Internet of Bio-Nano Things (IoBNT) is a networking paradigm that integrates living biological entities and synthetic micro/nanoscale devices—Bio-Nano Things (BNTs)—to enable molecular-level sensing, actuation, and communication within biological environments. The realization of IoBNT hinges on the development of BNT architectures that are simultaneously programmable, biocompatible, and capable of autonomous operation. Existing BNT paradigms—nanomaterial-based abiotic devices, passive molecular agents, and genetically engineered biosynthetic devices—each suffer from critical limitations: toxicity, lack of agency, or biosafety and metabolic burdens associated with genetic modification.
This paper introduces a fourth paradigm: transient hijacking of living cells via non-genetic cell surface engineering (NG-CSE). NG-CSE enables the functionalization of cell membranes with synthetic molecular machinery, imparting programmable interfaces for sensing, communication, and actuation without altering the genome. This approach merges the agency and biocompatibility of living cells with the programmability of nanotechnology, circumventing the liabilities of genetic engineering and foreign nanomaterials.
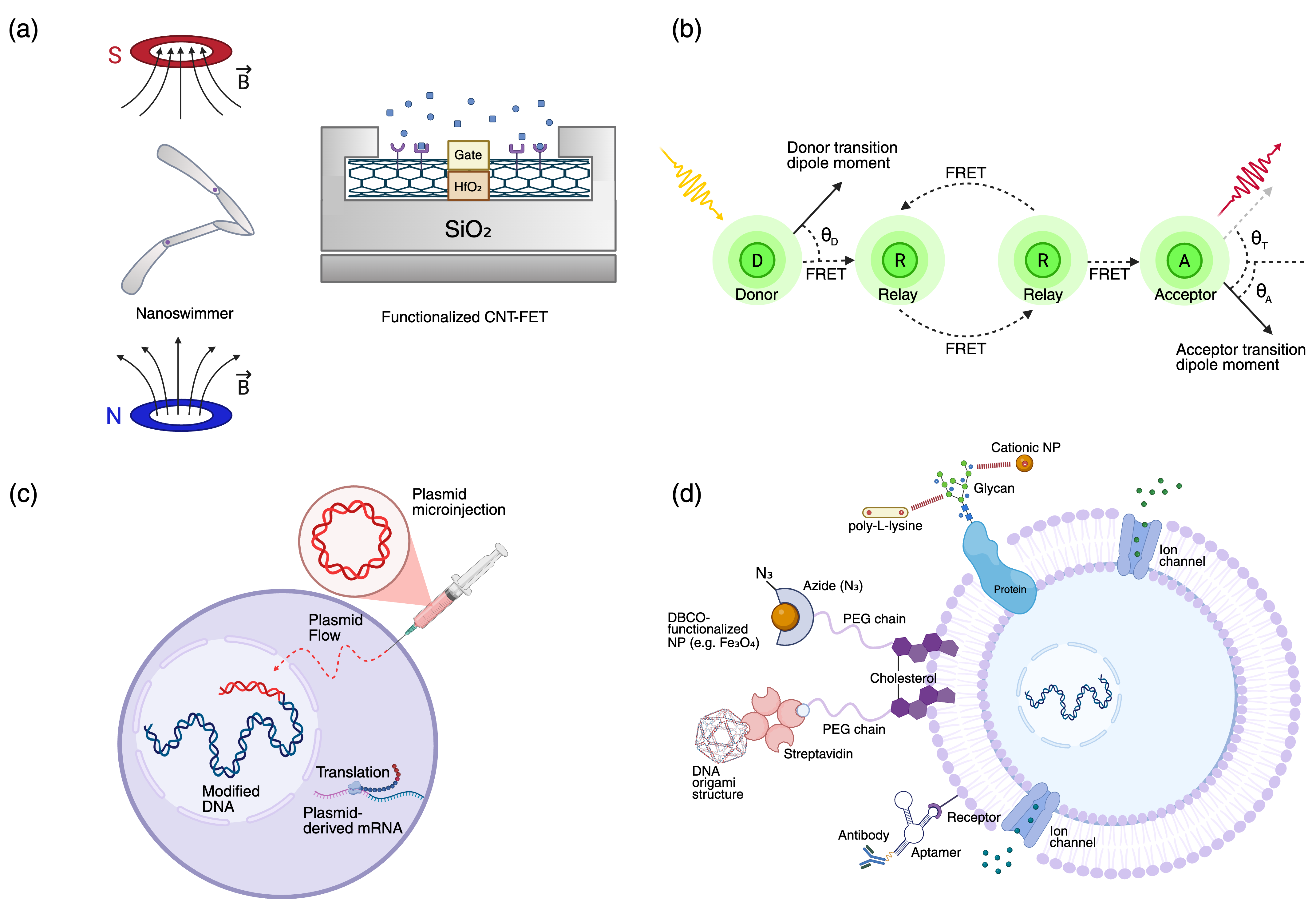
Figure 1: Comparative landscape of BNT architectures for IoBNT, highlighting the unique combination of agency and programmability in NG-CSE-enabled living cell BNTs.
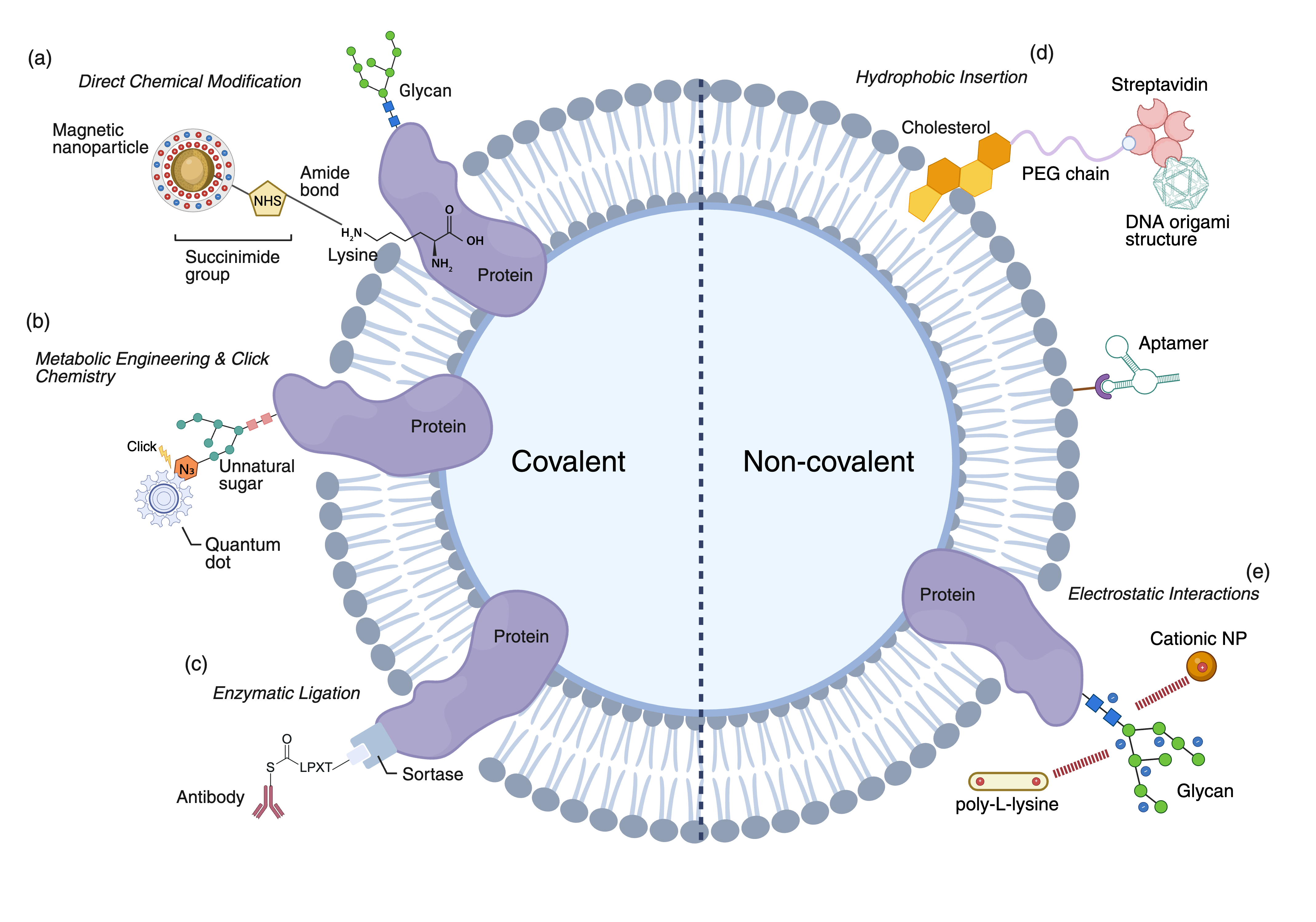
NG-CSE leverages a diverse set of engineering methods and functional materials to transform the cell membrane into a programmable interface. Engineering strategies are categorized by linkage type:
- Covalent strategies: Direct chemical modification (e.g., NHS-ester/amine, maleimide/thiol), metabolic glycoengineering with bioorthogonal click chemistry, and site-specific enzymatic ligation (e.g., Sortase A, glycosyltransferases) provide robust anchoring and spatial control.
- Non-covalent strategies: Hydrophobic insertion of lipid/cholesterol-anchored materials and electrostatic interactions enable rapid, reversible, and biocompatible functionalization.
Functional materials include:
Opportunities Enabled by NG-CSE in IoBNT
NG-CSE transforms living cells into dynamic, programmable BNTs, unlocking several key capabilities:
Programmable Sensing and Logic
Membrane-anchored DNA logic gates enable multi-signal processing and Boolean logic operations at the cell surface. For example, AND gates constructed from aptamers can trigger receptor assembly and downstream signaling only in the presence of specific combinations of molecular inputs, allowing for context-dependent actuation and communication.
Dynamic Network Topologies
Programmable adhesion molecules and DNA nanostructures facilitate the rational assembly of heterogeneous cell networks, enabling interkingdom communication and integration with abiotic devices. This supports the construction of distributed, multicellular systems with emergent collective behaviors.
Modulation of Mobility
Surface-tethered nanorobots and DNA devices can reversibly activate or halt cell migration by controlling integrin clustering or receptor dimerization. This allows for precise, real-time control of cellular agency, including motility and site-specific anchoring.
Bio-Cyber Interfacing
Surface-tethered nanoparticles enable remote actuation via external fields (magnetic, optical) and real-time environmental readout. For instance, magnetic nanoparticles allow for wireless control of mechanosensitive channels, while quantum dots and plasmonic particles facilitate optical sensing and actuation.
Energy Harvesting
Installation of photonic harvesters (e.g., quantum dot-protein hybrids) on the cell surface augments the cellular energy budget, enabling artificial photosynthesis and enhanced ATP production via FRET-mediated energy transfer.
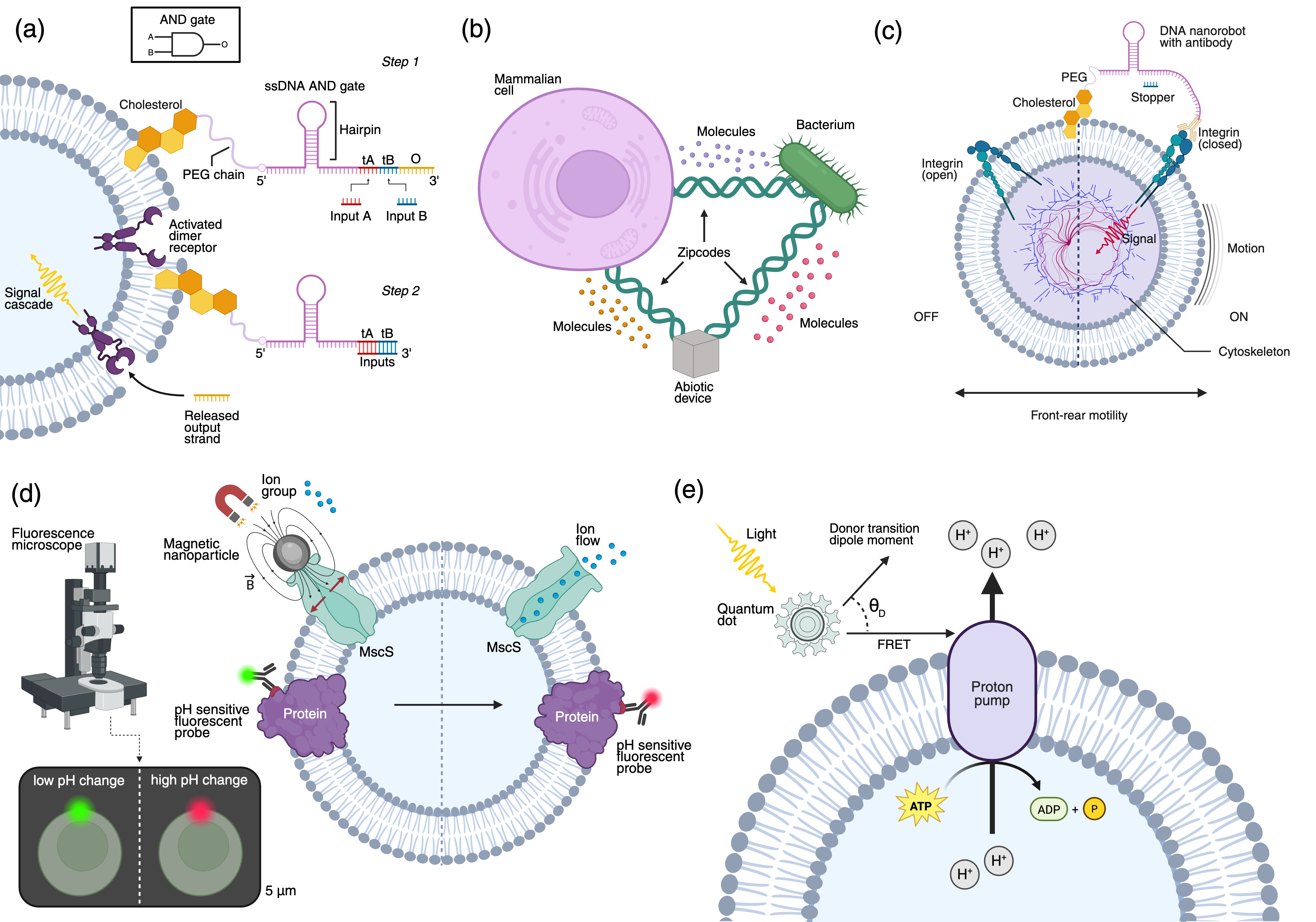
Figure 3: Key opportunities for IoBNT enabled by NG-CSE, including logic processing, network assembly, mobility control, bio-cyber interfacing, and energy harvesting.
IoBNT Applications Uniquely Enabled by NG-CSE
Circulating Sentinel Network for Continuous Liquid Biopsy
NG-CSE enables the in situ hijacking of red blood cells (RBCs) to create sentinel BNTs for continuous surveillance of circulating tumor DNA (ctDNA). Molecular engineering kits released by a programming depot functionalize RBC membranes with DNA logic gates. Activated sentinels are captured by a wearable microneedle patch, transducing biochemical detection events into electronic signals for real-time oncology monitoring.
Unconventional Computing with Rationally Designed Microphysiological Systems
NG-CSE allows for the rational design of biological reservoirs for unconventional computing. Heterogeneous cell populations are surface-engineered with DNA zip codes, directing the self-assembly of structured networks on micro-electrode arrays (MEA). This enables reproducible, high-dimensional wetware computation, moving beyond stochastic self-organization in organoid intelligence frameworks.
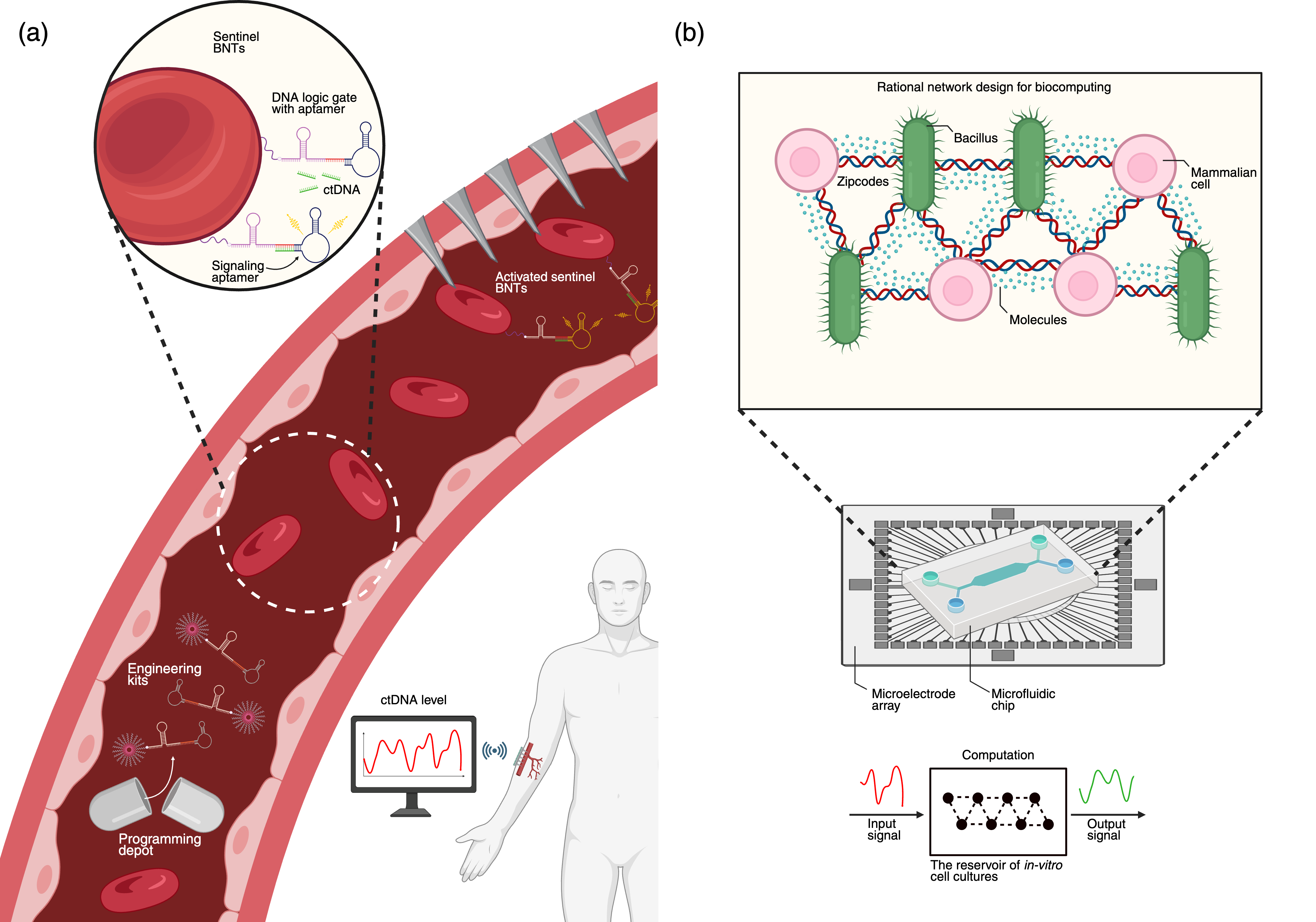
Figure 4: IoBNT applications enabled by NG-CSE, including circulating sentinel networks for liquid biopsy and rationally designed wetware computers.
Engineering and Modeling Challenges
Multi-Scale Channel Modeling
NG-CSE-enabled living BNTs are autonomous agents with complex, agential behaviors. Existing MC models are insufficient, necessitating multi-scale frameworks that integrate nanoscale component kinetics, membrane dynamics, and agent-based modeling (ABM) of population-level phenomena. This is essential for accurate prediction of communication channel characteristics and network reliability.
Agency-Aware Network Protocols
Network protocols must be redesigned to incorporate cellular agency as a primary routing metric. Routing decisions should consider metabolic state, motility, and chemotactic behavior, enabling biologically efficient and robust information transmission.
Lifecycle Management and In-Situ Reconfiguration
NG-CSE modifications are transient, subject to membrane turnover and internalization. BNT lifetime models must capture stochastic degradation processes, informing network maintenance and reliability. Dynamic covalent chemistry and stimuli-responsive materials enable in-situ reconfiguration, allowing BNTs to be reprogrammed post-deployment for adaptive IoBNT architectures.
Conclusion
NG-CSE represents a flexible and high-potential strategy for constructing programmable, biocompatible, and agency-enabled living BNTs for IoBNT. By transforming the cell membrane into a dynamic interface, NG-CSE bridges synthetic and biological domains, enabling advanced applications in diagnostics, computing, and therapeutics. Realizing this potential requires interdisciplinary advances in multi-scale modeling, agency-aware protocols, and lifecycle management. NG-CSE provides a robust foundation for the development of intelligent, adaptive, and seamlessly integrated bio-cyber systems in future IoBNT architectures.