- The paper presents a comprehensive deep learning framework that automates detection, segmentation, and Gleason Grade estimation for prostate cancer in mpMRI.
- The methodology employs a multi-channel 3D Retina U-Net architecture with rigorous pre-processing to enhance lesion detection and diagnostic accuracy.
- Results demonstrate improved sensitivity and specificity over traditional radiologist assessments, indicating strong potential for clinical integration.
Deep Learning for Prostate Cancer Detection in mpMRI
The paper "Deep Learning for fully automatic detection, segmentation, and Gleason Grade estimation of prostate cancer in multiparametric Magnetic Resonance Images" presents a comprehensive deep learning framework designed to enhance prostate cancer diagnosis through multiparametric MRI (mpMRI) analyses. By utilizing the Retina U-Net detection architecture, the authors aim to automate the detection, segmentation, and Gleason grade prediction of prostate cancer lesions, thereby improving diagnostic accuracy and consistency.
Introduction and Background
Prostate cancer remains the most common malignancy among males globally. The advent of mpMRI has significantly reshaped the diagnostic pathway in prostate cancer, offering enhanced glandular imaging that aids in better-targeted biopsies. However, interpreting mpMRI images is complex and often subjective, leading to variability in clinical outcomes. The challenge of manual interpretation opens avenues for computer-aided diagnostic (CAD) systems, which leverage machine learning to analyze imaging data with speed and accuracy.
The study outlines existing paradigms in CAD for prostate cancer detection, noting the shift from earlier statistical models to deep learning frameworks capable of handling complex image data and performing tasks beyond mere classification, such as lesion segmentation.
Methodology
The paper utilizes a dataset composed of mpMRIs from two distinct sources, ProstateX and IVO. It employs a multi-channel approach, integrating sequences such as T2-weighted, diffusion-weighted, and ADC maps to provide a comprehensive input for the model. A critical component of the preparation involves rigorous pre-processing to standardize and enrich the data, including automated zonal segmentation and sequence registration.











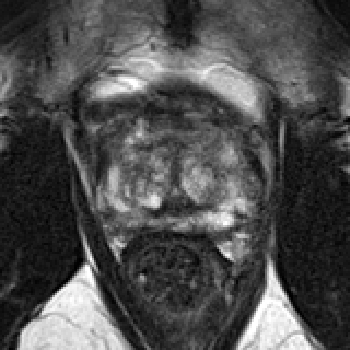










Figure 1: Final pre-processed image from a single patient (top: IVO, bottom: ProstateX). Channels (from left to right): T2, b400/b500, b800/b1000/b1400, ADC, Ktrans.
The proposed system utilizes a 3D Retina U-Net, combining the precision of Retina Net detection capabilities with U-Net's segmentation prowess. It is noteworthy for its efficiency in medical image processing, accommodating the number and scale of detections seamlessly across resolutions.



Figure 2: Automatic registration between T2 sequence (left) and ADC map (center: before, right: after) for a sample mpMRI.
Results
The model's efficacy is assessed through lesion-level and patient-level evaluations, utilizing soft thresholds for sensitivity and specificity. It showcases high AUC scores for the significance criterion of Gleason Grade Group ≥2, with the sensitivity and specificity metrics evidencing substantial improvements over traditional radiologist assessments. The model consistently outperformed expert interpretations in lesion detection tasks, demonstrating robustness across both datasets.










Figure 3: Output of the model evaluated on two IVO test patients. GGG0 (benign) BBs are not shown and only the highest-scoring BB is shown for highly overlapped detections (IoU >0.25).















Figure 4: Output of the model evaluated on three ProstateX test patients.
The validation on the ProstateX challenge set confirms its competitive performance, aligning closely with top contenders in the field, which typically involve manual ROI pre-selection instead of fully automated detection.
Discussion
The implications for clinical practice are substantial, with the potential for the system to assist in radiology workflows by reducing misinterpretation risks and expediting the diagnostic process. This research contributes to advancing CAD systems with fully independent lesion detection capabilities, paving the way for more extensive clinical trials.
The paper suggests potential directions for future AI systems in medicine, emphasizing the inclusion of diverse datasets to enhance model generalizability and reliability. The publicly available codebase encourages further development by other researchers aiming to refine or build upon the proposed model.
Conclusion
This paper presents a significant contribution to prostate cancer diagnosis through deep learning, showcasing the practical and theoretical potential of integrating advanced AI techniques in clinical settings. Future research could explore the application beyond oncology, employing similar methodologies for other complex image analysis tasks in medical diagnostics. This research highlights the transformational impact AI can have on medical imaging interpretation, improving accuracy and clinical outcomes in prostate cancer diagnosis.














































