- The paper demonstrates that computational models elucidate DBS mechanisms by regularizing neural firing within basal ganglia circuits.
- It applies Hodgkin-Huxley and multicompartmental models to predict optimal stimulation frequencies and electrode placements.
- The findings indicate potential for personalized DBS therapy with lower energy consumption and more targeted neural activation.
Computational Models Advance Deep Brain Stimulation for Parkinson's Disease
Overview of Deep Brain Stimulation (DBS) in Parkinson’s Disease
Deep Brain Stimulation (DBS) has emerged as a viable therapeutic option to alleviate motor symptoms associated with Parkinson’s Disease (PD), primarily targeting the basal ganglia (BG). Introduced in the late 20th century, DBS modulates aberrant neural signals through electrode-delivered electrical pulses without causing significant neural destruction akin to invasive procedures like pallidotomy. Despite its widespread application, the mechanism of action remains largely speculative. Understanding the underlying neurological circuits and their disturbances in PD is pivotal for advancing DBS efficacy. Incorporating computational models facilitates a deeper insight into the biological complexities and permits novel therapeutic explorations.
Anatomical and Functional Insights into Basal Ganglia (BG)
The BG consists of interconnected nuclei, namely the striatum (STR), globus pallidus (GP), subthalamic nucleus (STN), and substantia nigra (SN), playing crucial roles in motor control and decision-making. The classical BG model delineates two pathways: direct and indirect, with the STN emerging as a critical target for DBS due to its influential position within these motor circuitry loops.
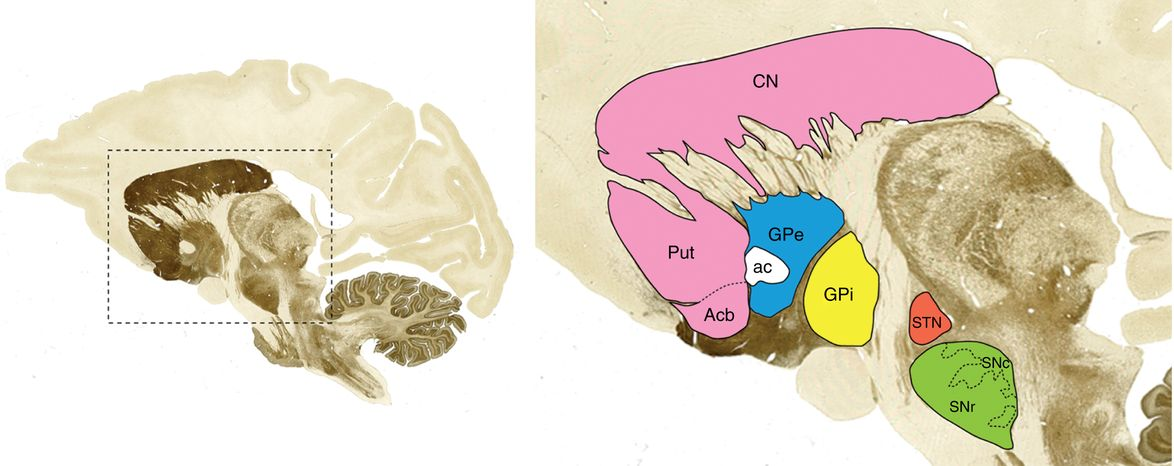
Figure 1: The location of basal ganglia along with its anatomy (monkey brain).
In PD, the pathological haLLMark includes the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), resulting in motor system suppression due to altered BG pathway dynamics. These changes induce increased oscillatory activity, particularly in the β frequency band (13-30 Hz), which is correlated with motor symptoms like bradykinesia and rigidity.
Mathematical Theoretical Models
Mechanistic Exploration: Neural Firing and Pathway Modulation
Rubin and Terman's seminal model in 2004 explored the impacts of STN high-frequency stimulation (HFS) on BG dynamics, particularly how oscillatory GPi output disrupts thalamocortical relay (TC) fidelity. Utilizing Hodgkin-Huxley type neurons, their model suggests that DBS alleviates PD symptoms by regularizing GPi outputs, thus restoring TC relay function.
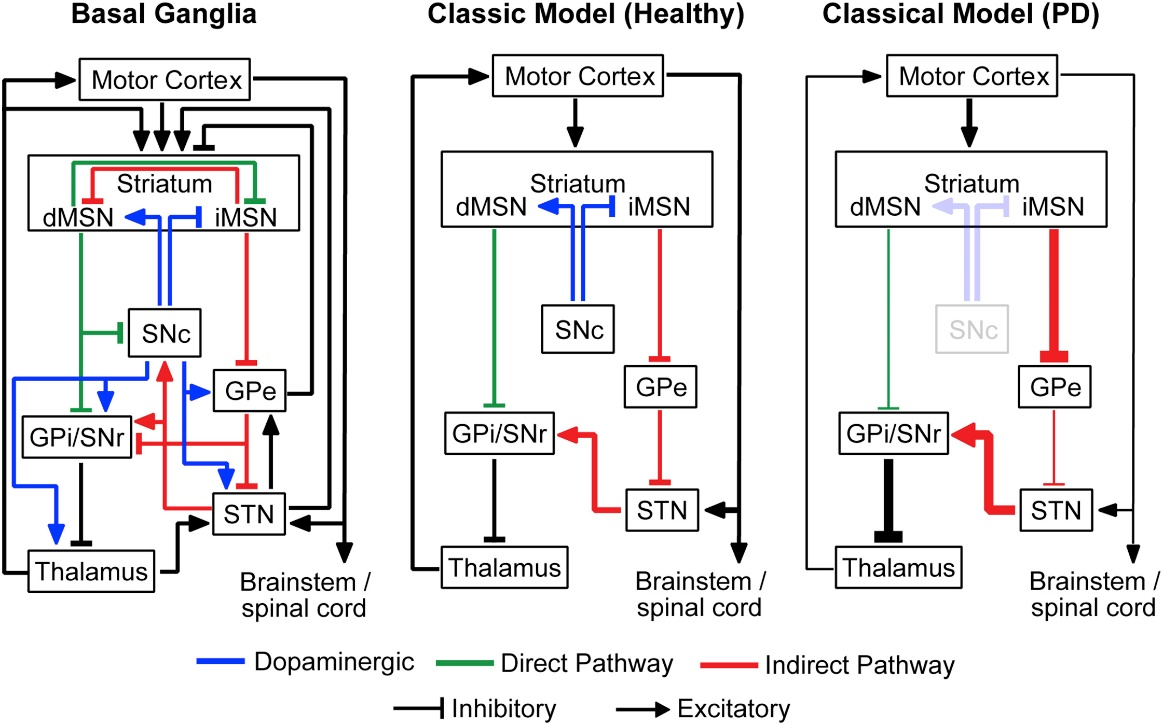
Figure 2: The classical model of basal ganglia activity pathways.
Subsequent models, such as McIntyre and Grill’s multicompartmental neuron model, highlighted DBS's dual action: inhibiting somatic activity while simultaneously stimulating axons, leading to “Informational Lesion.”
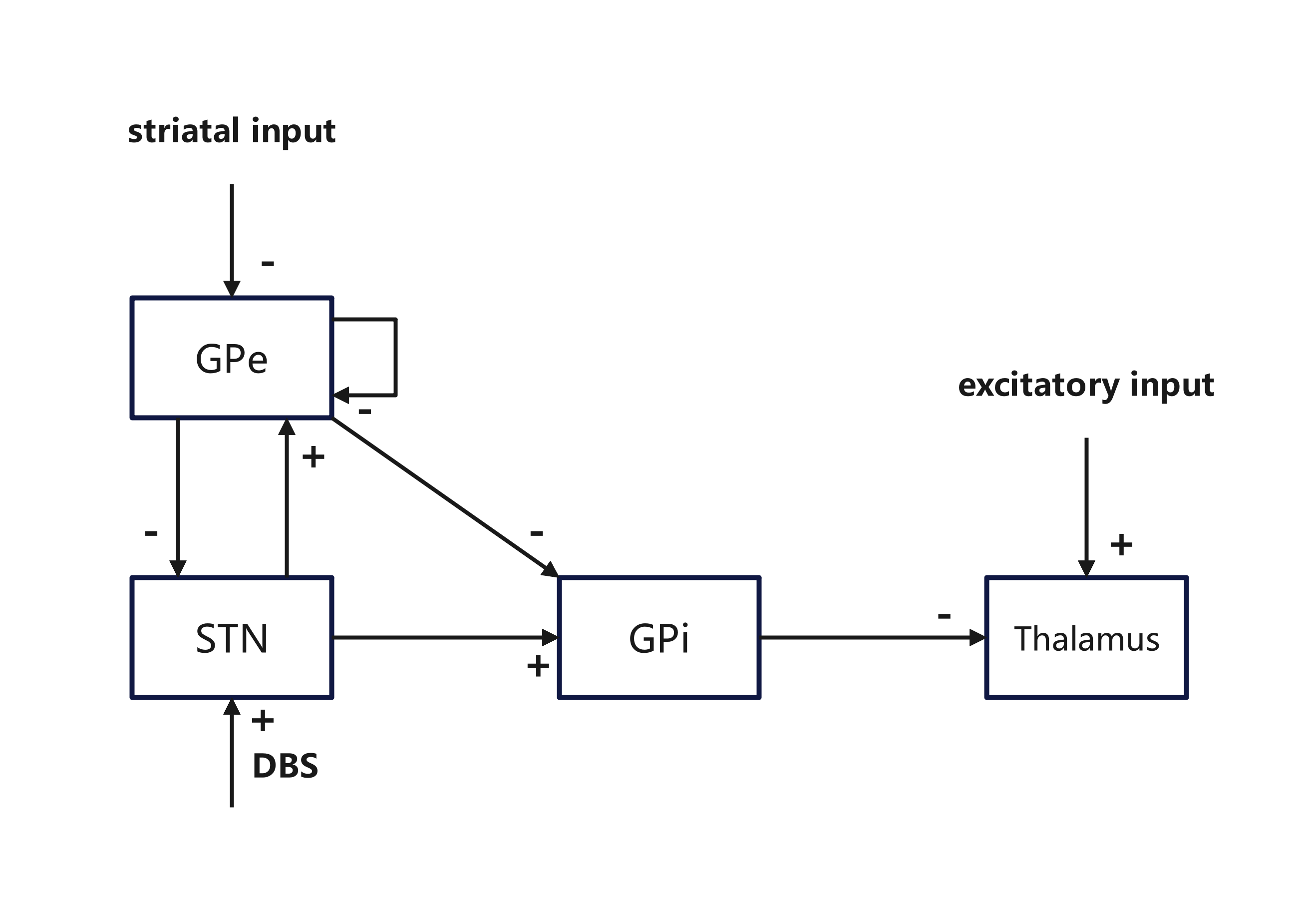
Figure 3: Structures in the Rubin and Terman model highlighting synaptic connections.
Targeting Alternative Pathways and Stimulating Patterns
Extensions of the original models analyzed various stimulation targets like GPe and GPi and their response to DBS frequencies. In particular, Humphries and Gurney's network-level simulations predicted STN's natural resonance frequency as an optimal therapeutic target, proposing that $100$ Hz is an effective clinical frequency for DBS.
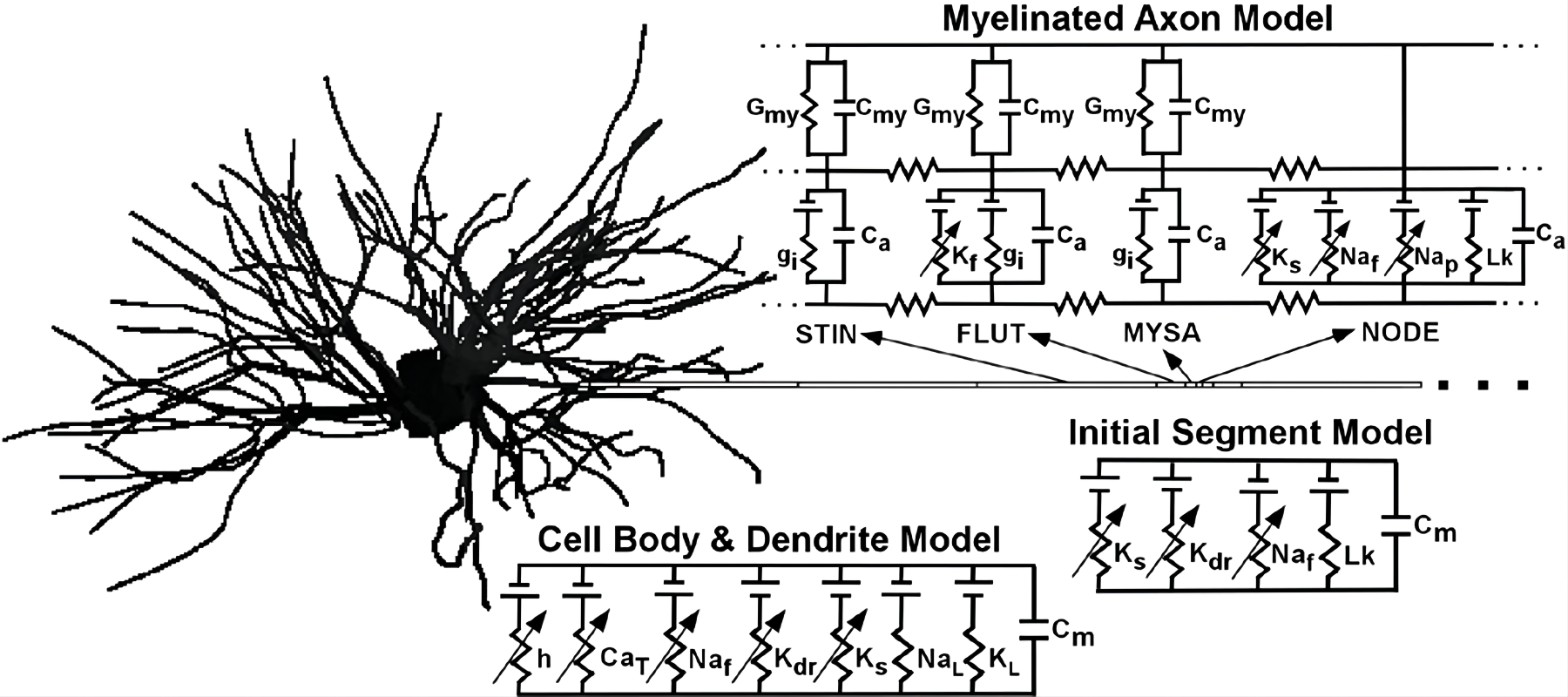
Figure 4: Structure of the hypothalamic pallidum network of McIntyre et al.
Experiments with non-regular stimulation patterns in computational models have suggested potential for enhancing DBS efficacy with lower energy consumption, which correlates with clinical observations.
Clinical Predictive Models
Patient-Specific Modeling and Optimization
Clinical predictive models integrate patient-specific anatomical data to simulate DBS effects, offering precision in electrode placement and stimulation parameterization to maximize therapeutic outcomes and minimize adverse effects.
Programs like Cicerone and LEAD-DBS provide visualizations aiding surgical planning by simulating volumes of tissue activation (VTA) for tailored DBS interventions.
Modeling has also driven innovations in electrode designs, such as segmented directional leads that offer refined control over stimulation fields, facilitating targeted therapy while reducing collateral effects.
Conclusion
Computational models act as crucial tools in the exploration and advancement of DBS for PD, elucidating mechanisms, testing hypotheses, and optimizing practical applications. Bridging the gap between theoretical models and clinical outcomes remains a vital endeavor for continued improvements in DBS therapy efficacy and safety. As the interface between computational neuroscience and medical practice strengthens, DBS models will evolve and integrate further into standard medical protocols, potentially offering insights into and solutions for a wider range of neurological conditions beyond traditional movement disorders.



