- The paper presents a comprehensive framework for personalized tES, highlighting subject-specific anatomical modeling and FEM-based electric field simulations.
- It details inverse optimization methods, including convex programming, genetic algorithms, and deep learning, to determine optimal stimulation parameters.
- The review emphasizes integrating multimodal neuroimaging and real-time feedback to refine models and overcome practical hardware and artifact challenges.
Introduction and Motivation
This paper provides a comprehensive review of computational modeling frameworks for personalized transcranial electrical stimulation (tES), emphasizing the critical role of individualized simulation and optimization in neuromodulation. The authors systematically dissect the pipeline from anatomical data acquisition to electric field simulation and stimulation parameter optimization, highlighting the necessity of subject-specific models to address inter-individual variability in neuroanatomy and neurophysiology. The review also covers the integration of multimodal neuroimaging and real-time brain-state feedback, which are essential for advancing precision neuromodulation in both research and clinical settings.
Forward Modeling: From Anatomy to Electric Field Simulation
The forward modeling process in tES involves constructing a subject-specific head model to predict the spatial distribution of electric fields induced by scalp-applied currents. The pipeline begins with high-resolution MRI-based tissue segmentation, followed by mesh generation and assignment of empirically derived conductivity values to each tissue compartment. The resulting volume conductor model is then used to solve Laplace’s equation under appropriate boundary conditions, typically via the finite element method (FEM), to obtain the electric field distribution.
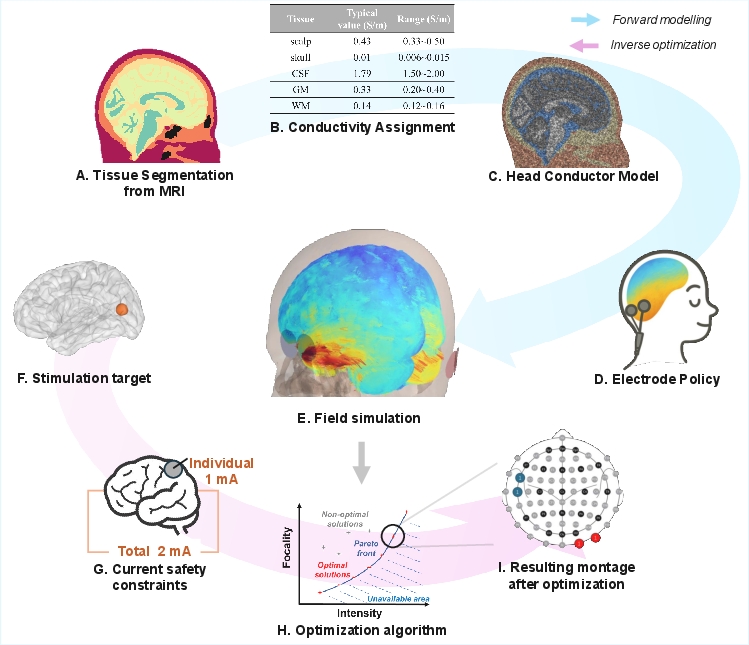
Figure 1: Overview of the forward modeling and inverse optimization pipeline for personalized tES, from MRI-based segmentation to electric field simulation and stimulation parameter optimization.
The review details the evolution from early spherical models to anatomically realistic, individualized simulations, enabled by advances in neuroimaging and high-performance computing. The authors discuss the trade-offs between FEM, boundary element method (BEM), and finite difference method (FDM), noting that FEM is preferred for its flexibility in handling complex, heterogeneous, and anisotropic tissue properties.
The paper also provides a critical comparison of widely used software tools, such as SimNIBS and ROAST, focusing on segmentation accuracy, mesh quality, and computational efficiency. The CHARM pipeline in SimNIBS 4 is highlighted for its robust segmentation of fifteen head tissues and superior performance across diverse imaging protocols.
Inverse Optimization: Algorithmic Personalization of Stimulation
Once the forward model is established, the challenge shifts to inverse optimization—systematically determining stimulation parameters (electrode positions, currents, waveforms) that maximize efficacy and specificity while adhering to safety and hardware constraints. The optimization problem is formalized using the leadfield matrix, which encodes the mapping from scalp currents to brain electric fields, and is solved with respect to biologically meaningful objective functions and safety constraints.
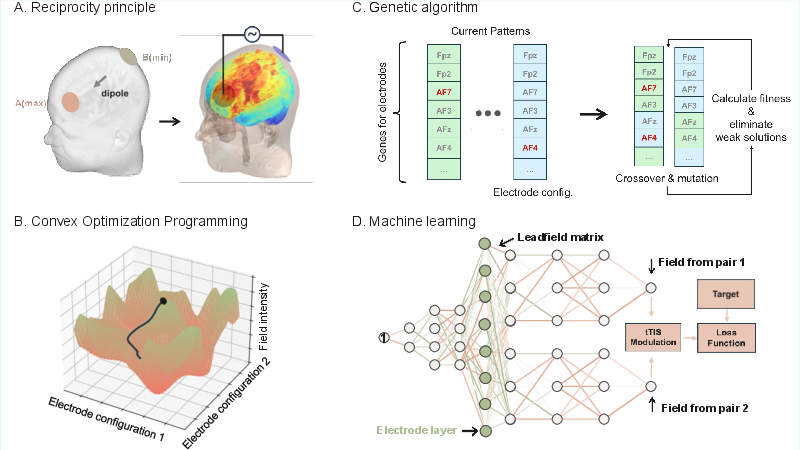
Figure 2: Schematic of major optimization strategies for tES: (A) Reciprocity principle, (B) Convex optimization, (C) Genetic algorithms, and (D) Deep learning-based approaches.
The review categorizes optimization algorithms into four main classes:
- Reciprocity Principle: Utilizes the symmetry of current flow to rapidly identify optimal electrode configurations for targeted stimulation.
- Convex and Linear Programming: Employs least-squares and directional maximization under convex constraints, forming the computational backbone of mainstream tools (e.g., SimNIBS, ROAST).
- Heuristic and Evolutionary Algorithms: Genetic algorithms and multi-objective evolutionary algorithms (e.g., MOVEA) are used for non-convex, multi-target, or highly constrained problems.
- Deep Learning: Neural networks are trained to generate optimal electrode currents, enabling flexible handling of complex, non-linear objectives.
The authors emphasize the intensity-focality trade-off and the need for multi-objective optimization, especially for deep or multi-target stimulation. They also note that the use of the leadfield matrix enables efficient evaluation of candidate solutions without repeated forward simulations.
Model Refinement: Multimodal Integration and Brain-State Feedback
A significant portion of the review is devoted to the refinement of computational models through the integration of multimodal neuroimaging and real-time brain-state feedback. The authors identify two primary axes of model updating:
- Anatomical and Biophysical Refinement: Incorporation of detailed tissue segmentation (e.g., bone subdivisions, meninges), subject-specific conductivity calibration (using sEEG or iEEG), and anisotropic conductivity tensors derived from diffusion MRI. These refinements are shown to substantially alter predicted electric field distributions, especially in deep or lesioned brain regions.
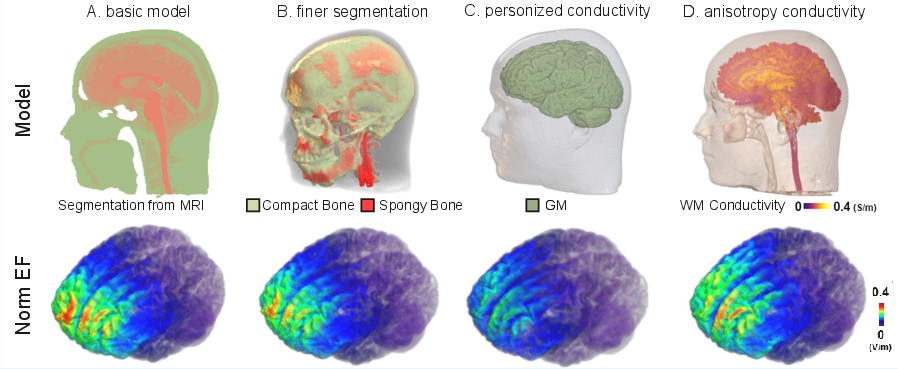
Figure 3: Impact of model updates on electric field norm: (A) standard model, (B) bone subdivision, (C) conductivity optimization, (D) anisotropy modeling.
- Dynamic, State-Dependent Optimization: The review highlights the limitations of treating the brain as a passive conductor and advocates for the integration of real-time neural signals (EEG, fMRI, sEEG) into the optimization loop. Theoretical frameworks from network controllability and control theory are proposed, where the brain is modeled as a dynamic system and stimulation is optimized to drive the system toward desired states. The authors discuss the use of hidden Markov models and closed-loop tES-fMRI paradigms to adapt stimulation in response to ongoing brain activity.
Limitations and Open Challenges
The review identifies several persistent challenges:
- Hardware-Model Integration: Real-world variability in electrode placement, contact quality, and device properties is not fully captured by current models.
- Artifact Management: Simultaneous tES-EEG is hampered by large stimulation artifacts, complicating real-time feedback and closed-loop control.
- Frequency and Orientation Effects: Most models neglect frequency-dependent tissue properties and the critical role of field orientation, particularly for tTIS and high-frequency protocols.
- Validation and Ground Truth: There is a lack of ground truth data for validating denoised neural signals and model predictions, especially during ongoing stimulation.
The authors argue that addressing these challenges will require the development of adaptive, data-driven modeling frameworks, improved artifact rejection techniques, and deeper integration of neuroimaging and electrophysiological data.
Implications and Future Directions
The reviewed advances in computational modeling for personalized tES have direct implications for both basic neuroscience and clinical neuromodulation. The ability to simulate and optimize electric field distributions at the individual level enables more precise targeting, reduced inter-subject variability, and improved efficacy in both research and therapeutic contexts. The integration of multimodal data and real-time feedback paves the way for adaptive, closed-loop neuromodulation systems.
From a theoretical perspective, the move toward dynamic, state-dependent optimization aligns tES research with contemporary systems neuroscience and control theory, offering a principled framework for understanding and manipulating brain network dynamics.
In the context of AI, the increasing use of machine learning for model refinement, artifact rejection, and optimization suggests a convergence between computational neuroscience and data-driven engineering. Future developments are likely to include reinforcement learning-based closed-loop controllers, large-scale data-driven conductivity estimation, and real-time adaptive stimulation protocols.
Conclusion
This review establishes a rigorous framework for computational modeling in personalized tES, spanning anatomical modeling, electric field simulation, algorithmic optimization, and dynamic model refinement. The synthesis of biophysical modeling, optimization theory, and multimodal data integration is essential for advancing precision neuromodulation. Continued progress will depend on addressing open challenges in model validation, artifact management, and real-world deployment, as well as leveraging advances in AI and control theory for adaptive, individualized brain stimulation.




