- The paper introduces Tippy, an AI-driven framework that automates the DMTA cycle to boost drug discovery efficiency.
- The multi-agent architecture—with Supervisor, Molecule, Lab, Analysis, and Report agents—ensures specialized task execution and seamless coordination.
- Integration with existing laboratory infrastructure and data systems supports automated record updates and robust regulatory compliance.
Agentic AI for Accelerated Drug Discovery
This paper introduces Tippy, an agentic AI framework designed to accelerate drug discovery through laboratory automation. The system employs a multi-agent approach to manage the Design-Make-Test-Analyze (DMTA) cycle, a core process in pharmaceutical research. The paper posits that by using specialized AI agents, Tippy can enhance workflow efficiency, decision-making speed, and cross-disciplinary coordination.
Tippy's Multi-Agent Architecture
Tippy's architecture consists of five specialized agents: Supervisor, Molecule, Lab, Analysis, and Report, overseen by a Safety Guardrail (Figure 1). Each agent is designed to excel in a specific phase of the drug discovery pipeline, using tailored tools and expertise.
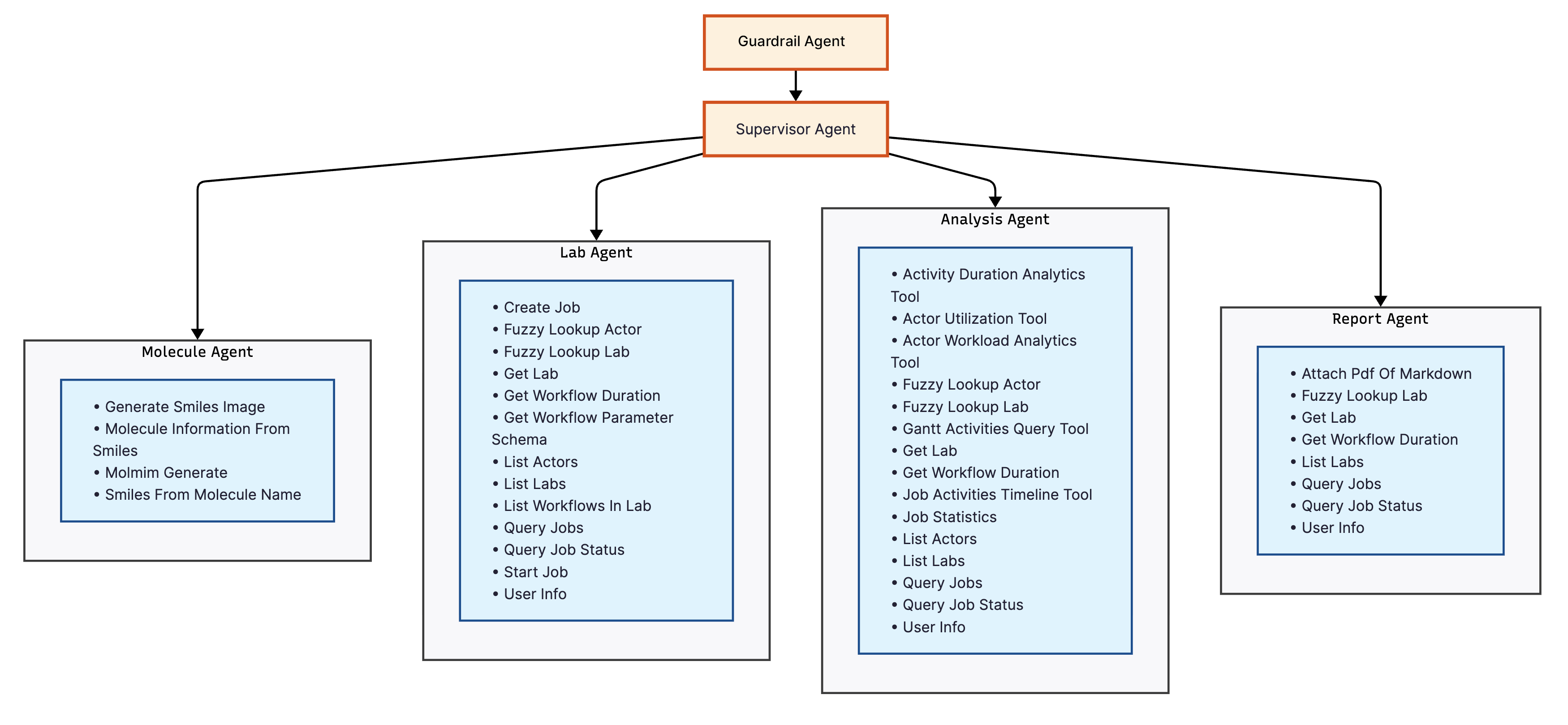
Figure 1: Tippy's multi-agent architecture showing specialized agents (Supervisor, Molecule, Lab, Analysis, Report, and Safety Guardrail) with their associated tools for laboratory automation and drug discovery workflows.
- The Supervisor Agent acts as the central coordinator, managing workflows and providing a primary interface for human researchers.
- The Molecule Agent focuses on computational chemistry tasks, such as generating molecular structures and optimizing drug-likeness properties.
- The Lab Agent manages laboratory automation and instrumentation, handling HPLC analysis and synthesis procedures.
- The Analysis Agent processes job performance data and extracts statistical insights from laboratory workflows.
- The Report Agent generates summary reports and detailed scientific documentation from experimental data.
- The Safety Guardrail Agent validates all user requests for potential safety violations.
This specialization allows each agent to operate autonomously within its domain while maintaining seamless coordination through the supervisor.
DMTA Cycle Implementation
Tippy automates the DMTA cycle by integrating these agents into a cohesive workflow. In the Design phase, the Molecule Agent generates and optimizes molecular structures. The Lab Agent then manages the Make phase by creating synthesis jobs and coordinating HPLC analysis. During the Test phase, the Analysis Agent processes incoming data in real-time, while the Lab Agent orchestrates experimental execution. Finally, in the Analyze phase, the Analysis Agent extracts insights and feeds this information back to the Molecule Agent for the next design iteration (Figure 2).
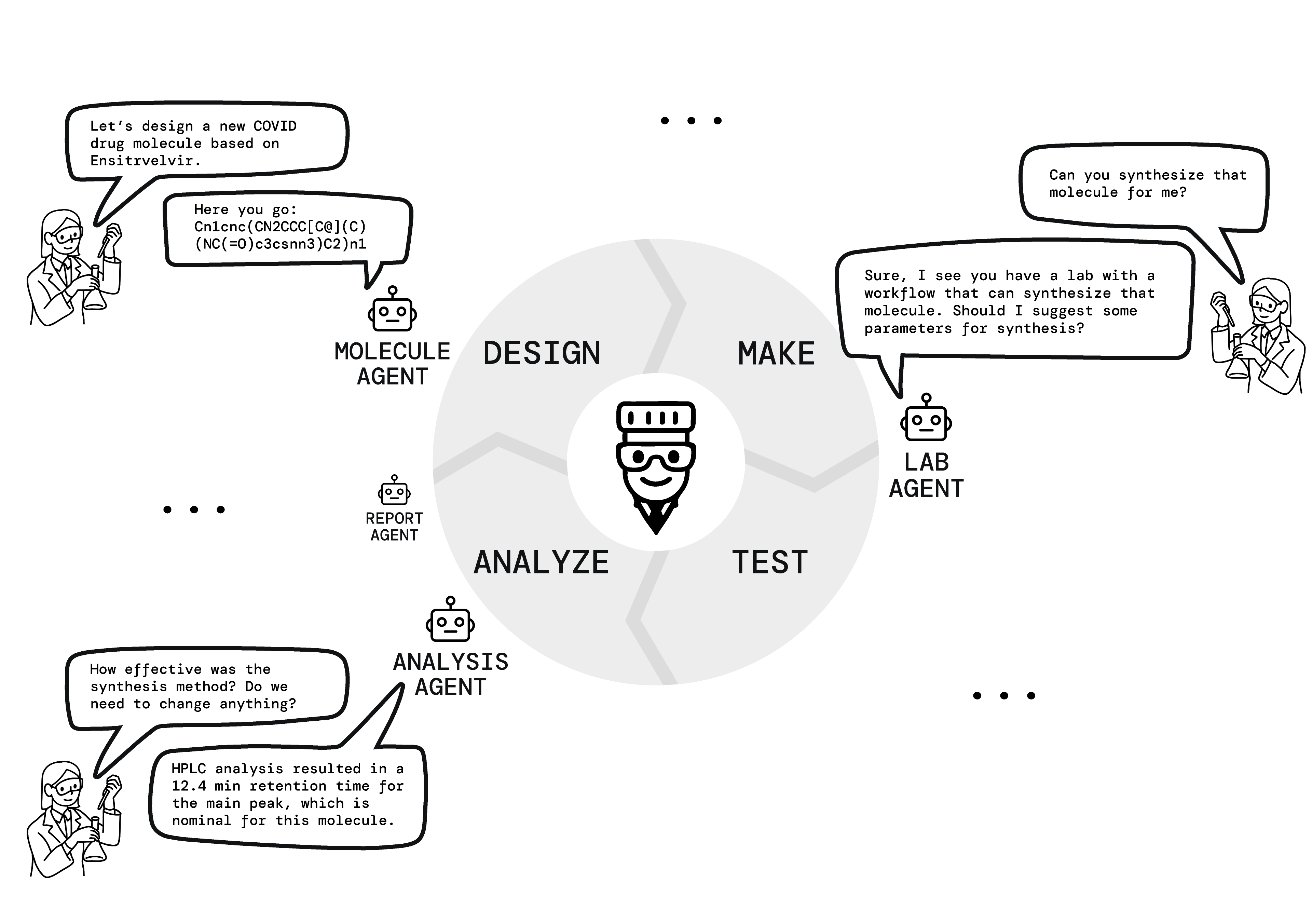
Figure 2: It shows how Tippy's agents work with researchers in each step of the DMTA cycle. The Molecule Agent helps with design, the Lab Agent manages synthesis and testing, the Analysis Agent processes results, and the Report Agent documents findings, creating a continuous feedback loop for drug discovery.
This closed-loop system mirrors the iterative refinement process used by human medicinal chemists, but with increased speed and efficiency.
Coordination and Collaboration Mechanisms
Effective coordination and collaboration between agents are crucial for the success of Tippy. The framework employs several mechanisms to ensure seamless cooperation:
- A hierarchical coordination structure, with the Supervisor Agent providing overall direction and conflict resolution.
- Dynamic handoff mechanisms, allowing agents to transfer control smoothly as workflows progress.
- Shared knowledge bases, ensuring that all agents have access to relevant information.
- Collaborative decision-making protocols, allowing multiple agents to contribute to complex decisions.
These mechanisms enable Tippy to function as a cohesive unit, optimizing the DMTA cycle.
Integration with Laboratory Infrastructure
Tippy is designed to integrate seamlessly with existing laboratory infrastructure, data systems, and workflows. The system uses the Model Control Protocol (MCP) to provide a standardized interface for AI agents to interact with laboratory systems and data resources. It also integrates with LIMS, ELNs, and analytical instrument data systems through standardized APIs and data formats. This compatibility ensures that Tippy can access historical data, update records automatically, and coordinate with existing quality control and regulatory compliance systems.
Conclusions and Implications
Tippy represents a significant advancement in drug discovery research, shifting from manual coordination to intelligent orchestration by autonomous AI agents. The multi-agent framework demonstrates that specialized AI systems can automate routine tasks, reason about complex scientific problems, and accelerate the DMTA cycle while maintaining scientific rigor. The paper suggests that the success of agentic AI opens opportunities for enterprise-scale implementation, enabling coordination of multiple DMTA cycles and integration with business intelligence platforms. Future development should focus on enhancing human expertise rather than replacing it, maintaining human responsibility for critical decisions while providing transparency for regulatory confidence. Ultimately, the collaboration between human and artificial intelligence offers significant potential for developing therapeutics more efficiently while maintaining the quality and safety standards essential for patient care.

